Assessment of Adherence to Consolidated Standards of Reporting Trials 2010 Guidelines of Randomized Controlled Trials Published in an Indian and International Pharmacology Journal From 2019 to 2023
- PMID: 40225544
- PMCID: PMC11990665
- DOI: 10.7759/cureus.80450
Assessment of Adherence to Consolidated Standards of Reporting Trials 2010 Guidelines of Randomized Controlled Trials Published in an Indian and International Pharmacology Journal From 2019 to 2023
Abstract
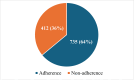
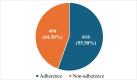
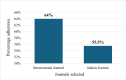
Randomized controlled trials (RCTs) are considered the gold standard in clinical research, providing the highest level of evidence for the effectiveness of healthcare interventions. However, the validity and utility of RCTs depend on the quality of their design, conduct, and reporting. The purpose of this review was to assess the adherence of RCTs published in Indian and international pharmacology journals to the Consolidated Standards of Reporting Trials (CONSORT) statement. RCTs published from 2019 to 2023 from one Indian and one international pharmacology journal were assessed using the CONSORT 2010 checklist, and the items were assigned as "present" or "absent." Data was analyzed using descriptive statistics, and chi-square and Fisher's exact tests were used for categorical data. A total of 61 articles were analyzed, out of which 31 and 30 articles belonged to international and Indian journals, respectively. RCTs published in international journals consistently showed higher adherence rates compared to Indian journals, with statistically significant differences for several checklist items, including trial design description (31, 100%), intervention details (31, 100%), and reporting of harms (27, 87%) (p < 0.05). While Indian journals performed better on points like additional analyses (19, 63.3%) and recruitment dates (23, 76.6%). Overall, the international journal demonstrated significantly higher overall adherence to CONSORT guidelines compared to the Indian journal (p < 0.05). The international journal exhibited greater overall adherence than the Indian journal, with the difference being statistically significant (p < 0.05). The overall reporting was suboptimal. Adherence should be improved further, and the journals should ensure the compliance of authors and reviewers with the standard reporting guidelines.
Keywords: chi-square test; clinical research; consolidated standards of reporting trials; fisher’s exact test; reporting guidelines.
Copyright © 2025, Hotwani et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Publication guidelines need widespread adoption. Larson EL, Cortazal M. J Clin Epidemiol. 2012;65:239–246. - PubMed
-
- An assessment of the compliance of randomized controlled trials published in two high impact journals with the CONSORT statement. Susvirkar A, Gada P, Figer B, Thaker S, Thatte UM, Gogtay NJ. Natl Med J India. 2018;31:79–82. - PubMed
Publication types
LinkOut - more resources
Full Text Sources