Nanocatalytic system releases overloaded zinc ions and ROS to induce Znproptosis and interrupt cell cycle through inhibiting Akt/mTOR pathway
- PMID: 40225560
- PMCID: PMC11984402
- DOI: 10.7150/thno.107025
Nanocatalytic system releases overloaded zinc ions and ROS to induce Znproptosis and interrupt cell cycle through inhibiting Akt/mTOR pathway
Abstract
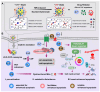
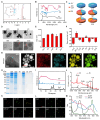
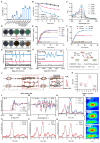
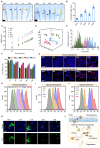
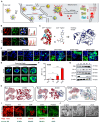
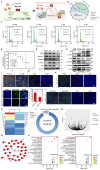
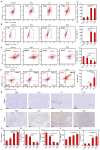
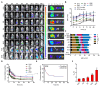
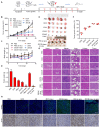
Background: Traditional programmed cell death, including ferroptosis, cuproptosis, and apoptosis, has demonstrated excellent anti-tumor effects and declared their complete mechanisms, however, the zinc ion-mediated tumor inhibiting mechanisms remain insufficiently explored. In this study, a self-generated oxygen nanocatalytic system (ZnO@COF@EM, ZCE) was developed to stimulate cascade amplified effect (CAE) of reactive oxygen species (ROS) generation leading to Znproptosis. The underlying Znproptosis mechanism to disrupt mitochondrial (Mito) metabolism was also investigated. Methods: Specifically, the principle of Znproptosis caused by accumulated zinc and ROS, which served as key factors, was declared through western blot analysis and genetic testing. The mechanism of generated ROS (·OH and 1O2) under NIR irradiation by ZCE was detected by UV scanning curves, confocal laser scanning microscopy (CLSM) images, and density functional analysis. The injury condition of Fe-S protein of mitochondria metabolism, which triggered Znproptosis with FDX2/LIAS pathway by zinc and ROS, was examined by PCR test and MTT assay. Notably, a Mito-targeting strategy for ZCE was proposed by using molecular docking technology, wherein Zn2+ was recognized by zinc finger proteins (ZFPs) with the Mito. Results: ZCE, along with CAE, produced abundant ROS (2.42-time more than control group). At the quantum chemical level, the CAE mechanism was associated with a narrower highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) gap and increased electronic energy motion within ZCE, which prolonged the excited triplet state (ETS). At the gene level, Znproptosis was achieved by regulating FDX2 and ZIP7 proteins to damage Fe-S protein. The cell cycle was interrupted by Chk2/Cdc25C/Cdc2 and Chk2/p21/Cyclin B1 pathways, leading to the arrest of G1/S and G2/M phases of the cell cycle and inhibition of the Akt/mTOR signaling pathway. Moreover, Znproptosis induced by overloading zinc ions and ROS resulted in a significant antitumor effect (up to 83.81%). Conclusion: Hence, the research reveals a detailed Znproptosis mechanism in nanocatalytic system. Through regulating FDX2/LIAS pathway, Znproptosis could improve the death rate of mitochondria by decreasing the production of Fe-S protein, contributing to advancements in the field of antitumor therapy.
Keywords: Akt/mTOR pathway; FDX2/LIAS pathway; Znproptosis; cascade amplificated effect; nanocatalyst.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Evaluating the in vitro and in vivo effects of carvacrol zinc oxide quantum dots in breast cancer.J Biomater Sci Polym Ed. 2025 Apr;36(6):796-815. doi: 10.1080/09205063.2024.2429325. Epub 2024 Dec 3. J Biomater Sci Polym Ed. 2025. PMID: 39625392
-
Fabrication of bimetallic Ag@ZnO nanocomposite and its anti-cancer activity on cervical cancer via impeding PI3K/AKT/mTOR pathway.J Trace Elem Med Biol. 2024 Jul;84:127437. doi: 10.1016/j.jtemb.2024.127437. Epub 2024 Mar 20. J Trace Elem Med Biol. 2024. PMID: 38564977
-
Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway.Drug Des Devel Ther. 2015 Mar 12;9:1555-84. doi: 10.2147/DDDT.S74197. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25792811 Free PMC article.
-
Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines.Biochem Biophys Res Commun. 2017 Dec 9;494(1-2):263-269. doi: 10.1016/j.bbrc.2017.10.042. Epub 2017 Oct 9. Biochem Biophys Res Commun. 2017. PMID: 29024631
-
Albanol B from Mulberries Exerts Anti-Cancer Effect through Mitochondria ROS Production in Lung Cancer Cells and Suppresses In Vivo Tumor Growth.Int J Mol Sci. 2020 Dec 14;21(24):9502. doi: 10.3390/ijms21249502. Int J Mol Sci. 2020. PMID: 33327489 Free PMC article. Review.
References
-
- Li Z, Ding B, Li J, Chen H, Zhang J, Tan J. et al. Multi-Enzyme Mimetic MoCu Dual-Atom Nanozyme Triggering Oxidative Stress Cascade Amplification for High-Efficiency Synergistic Cancer Therapy. Angewandte Chemie International Edition. 2025;64:e202413661. - PubMed
-
- Cheng J, Li L, Jin D, Dai Y, Zhu Y, Zou J. et al. Boosting Ferroptosis Therapy with Iridium Single-Atom Nanocatalyst in Ultralow Metal Content. Advanced Materials. 2023;35:2210037. - PubMed
-
- Zhang L, Qiu M, Wang R, Li S, Liu X, Xu Q. et al. Monitoring ROS Responsive Fe3O4-based Nanoparticle Mediated Ferroptosis and Immunotherapy via 129Xe MRI. Angewandte Chemie International Edition. 2024;63:e202403771. - PubMed
-
- Li W, Xiao Y, Guo G, Peng J, Zhu N, Chen Z, Cuprous oxide nanocomposites with photothermal (PTT) and chemical dynamics (CDT) effects induce cuproptosis in breast cancer using the strategy of increasing inflow and reducing outflow. Nano Today. 2024. 56.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

