A brain-accessible peptide modulates stroke inflammatory response and neurotoxicity by targeting BDNF-receptor TrkB-T1 specific interactome
- PMID: 40225562
- PMCID: PMC11984388
- DOI: 10.7150/thno.111272
A brain-accessible peptide modulates stroke inflammatory response and neurotoxicity by targeting BDNF-receptor TrkB-T1 specific interactome
Abstract
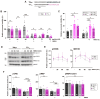
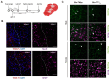
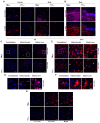
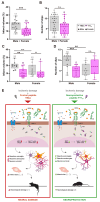
Glia reactivity, neuroinflammation and excitotoxic neuronal death are central processes to ischemic stroke and neurodegenerative diseases, altogether a leading cause of death, disability, and dementia. Given the high incidence of these pathologies and the limited efficacy of current treatments, developing brain-protective therapies that target both neurons and glial cells is a priority. Truncated neurotrophin receptor TrkB-T1, a protein produced by these cell types, plays relevant roles in excitotoxicity and ischemia. We hypothesized that interactions mediated by isoform-specific TrkB-T1 sequences might contribute to neurotoxicity and/or reactive gliosis, thus representing potential therapeutic targets. Methods: We designed cell-penetrating peptides containing TrkB-T1 isoform-specific sequences to: 1) characterize peptide delivery into rat primary cortical cultures and mice brain cortex; 2) isolate and identify the isoform interactome in basal and in vitro excitotoxic conditions; 3) analyze peptide effects on neuroinflammation and neurotoxicity using primary cultures subjected to excitotoxicity or in vivo in a mouse model of ischemia. Results: We identify here the TrkB-T1-specific interactome, poorly described to date, and demonstrate that interference of these protein-protein interactions using brain-accessible TrkB-T1-derived peptides can reduce reactive gliosis and decrease excitotoxicity-induced damage in cellular and animal models of stroke, where treatment reduces the infarct volume in male and female mice. Conclusions: The crucial role of TrkB-T1 in modulating microglia and astrocyte reactivity indicates that isoform-derived peptides hold promise for the development of therapies for human stroke and other excitotoxicity-associated pathologies.
Keywords: cell-penetrating peptides; excitotoxicity; inflammatory response; interactome; neurodegeneration; neuroprotection.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

