A new nano-encapsulated TSPO ligand reduces neuroinflammation and improves cognitive functions in Alzheimer's disease model
- PMID: 40225565
- PMCID: PMC11984405
- DOI: 10.7150/thno.106083
A new nano-encapsulated TSPO ligand reduces neuroinflammation and improves cognitive functions in Alzheimer's disease model
Abstract
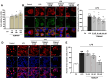
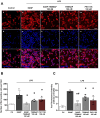
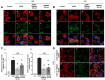
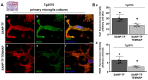
Rationale: The translocator protein 18 kDa (TSPO) is mainly expressed on the outer mitochondrial membrane and is implicated in inflammation, cell survival, and proliferation. TSPO expression in activated microglia is upregulated in Alzheimer's disease (AD), representing both a biomarker and therapeutic target for neuroinflammation. Methods: We synthesized a new TSPO ligand, TEMNAP, a hybrid of temazepam, a compound well known for its ability to bind TSPO, and naproxen, a drug with anti-inflammatory properties that is potentially useful to mitigate neuroinflammation. TEMNAP was encapsulated in a self-assembling nanoparticle transferrin-targeted (SANP-TF-TEMNAP) for brain delivery. The effectiveness of TEMNAP in mitigating inflammatory processes and cognitive behavior was investigated in genetically modified Tg2576 mice, a model of Alzheimer's disease. Its effect on neuroinflammation has also been explored in lipopolysaccharide-activated BV2 microglial cells. Results: SANP-TEMNAP significantly reduced the expression of proinflammatory markers in activated microglia, and this effect was abrogated by TSPO silencing. More importantly, TEMNAP was mass-spectrometrically detected in the hippocampus and cortex of Tg2576 mice after SANP-TF-TEMNAP intraperitoneal administration, preventing hippocampal neuroinflammation and improving cognitive function. Conclusions: These results emphasize the following: (i) the role of transferrin-conjugated self-assembling nanoparticles (SANP-TF) as CNS nanovectors, and (ii) the potential therapeutic effectiveness of peripherally administered SANP-TF-TEMNAP in preventing neuroinflammation associated with cognitive decline.
Keywords: Alzheimer's disease; TSPO; nanovectors; neurodegeneration; neuroinflammation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17:157–72. - PubMed
-
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N. et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–88. - PubMed
-
- Gargiulo S, Anzilotti S, Coda AR, Gramanzini M, Greco A, Panico M. et al. Imaging of brain TSPO expression in a mouse model of amyotrophic lateral sclerosis with (18)F-DPA-714 and micro-PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:1348–59. - PubMed
-
- Coda AR, Anzilotti S, Boscia F, Greco A, Panico M, Gargiulo S. et al. In vivo imaging of CNS microglial activation/macrophage infiltration with combined [(18)F]DPA-714-PET and SPIO-MRI in a mouse model of relapsing remitting experimental autoimmune encephalomyelitis. Eur J Nucl Med Mol Imaging. 2021;48:40–52. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

