The basolateral amygdala-anterior cingulate cortex circuit contributes to postherpetic neuralgia-anxiety comorbidity
- PMID: 40225572
- PMCID: PMC11984391
- DOI: 10.7150/thno.111130
The basolateral amygdala-anterior cingulate cortex circuit contributes to postherpetic neuralgia-anxiety comorbidity
Abstract
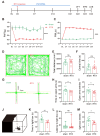
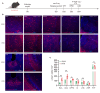
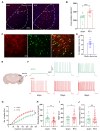
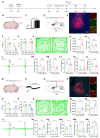
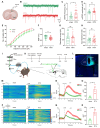
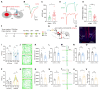
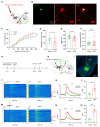
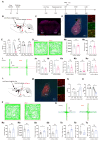
Background: Postherpetic neuralgia (PHN) causes chronic pain and emotional dysfunction, but its underlying mechanisms are unknown. Methods: We first compared the structural and functional magnetic resonance imaging (MRI) of PHN-anxiety patients with healthy controls (HCs). Then, we created a PHN comorbid anxiety-like model by injecting resiniferatoxin (RTX) intraperitoneally and used Fos-CreER::Ai9 mice to validate brain regions with volume differences in MRI. Furthermore, we combined behavioral experiments with electrophysiology, viral tracing, in vivo fiber-photometry, optogenetics, and chemogenetics, to analyze the role of the basolateral amygdala (BLA)-anterior cingulate cortex (ACC) circuit in PHN comorbid anxiety-like mice multi-dimensionally. Results: According to neuroimages, patients with PHN-anxiety comorbidity have decreased amygdala volume and decreased functional connection (FC) of the BLA and ACC. Furthermore, we created a PHN comorbid anxiety-like model by injection of RTX intraperitoneally, and these mice showed dysesthesia and anxiety-like behaviors 3 weeks after RTX injection. Then, we discovered that BLA and ACC were related to PHN comorbid anxiety-like behaviors using Fos-CreER::Ai9 mice. Immunohistochemistry and electrophysiology revealed enhanced activation of BLA glutamatergic (BLAGlu) neurons in PHN comorbid anxiety-like mice. Opto/chemogenetic activating BLAGlu neurons aggravated pain threshold in PHN comorbid anxiety-like mice. Inhibiting BLAGlu alleviates mechanical nociception, thermal hyperalgesia, and anxiety-like behavior. Moreover, the elevated excitability of BLAGlu neurons resulted in increased excitatory inputs to the ACC. Selective activation or inhibition of the BLAGlu-ACC pathway exacerbated or alleviated the pain and anxiety behavior, respectively. Conclusion: Findings in this study will provide new insight for understanding the central pathomechanism underlying PHN-anxiety comorbidity, as well as serve as solid theoretical underpinnings for the management of PHN.
Keywords: Anterior cingulate cortex; Basolateral amygdala; Neural circuit; Postherpetic neuralgia; fMRI.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Saguil A, Kane S, Mercado M, Lauters R. Herpes Zoster and Postherpetic Neuralgia: Prevention and Management. Am Fam Physician. 2017;96:656–63. - PubMed
-
- Sun X, Wei Z, Lin H, Jit M, Li Z, Fu C. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: Data from an integrated health care network. J Infect. 2021;82:253–60. - PubMed
-
- Asada H. Recent topics in the management of herpes zoster. J Dermatol. 2023;50:305–10. - PubMed
-
- Du J, Sun G, Ma H, Xiang P, Guo Y, Deng Y. et al. Prevalence and Risk Factors of Anxiety and Depression in Patients with Postherpetic Neuralgia: A Retrospective Study. Dermatology. 2021;237:891–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

