Improved optogenetic modification of spiral ganglion neurons for future optical cochlear implants
- PMID: 40225583
- PMCID: PMC11984395
- DOI: 10.7150/thno.104474
Improved optogenetic modification of spiral ganglion neurons for future optical cochlear implants
Abstract
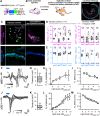
Optogenetic stimulation has become a promising approach for restoring lost body function. For example, partial restoration of vision has been achieved in a blind patient and preclinical proof-of-concept has been demonstrated for optogenetic hearing restoration. In preparation for clinical translation of hearing restoration, efficient and safe optogenetic modification of spiral ganglion neurons (SGNs) in the mature cochlea remained to be developed. Methods: Here, we established microcatheter-based administration of adeno-associated virus (AAV) into scala tympani of the cochlea of Mongolian gerbils and compared it to the previously developed direct AAV-injection into the spiral ganglion. We probed the potential of AAV-PHP.S to express channelrhodopsins (ChRs) under the control of the human synapsin promotor in mature SGNs of hearing and deafened gerbils. Results: Using the microcatheter approach, but not with the AAV-modiolus injection, we achieved reliable ChR expression in SGN enabling optogenetic stimulation of the auditory pathway in 80% of the treated animals. Yet, the efficiency of SGN transduction was modest with only ~30% ChR-expressing SGNs. Moreover, we encountered off-target expression in hair cells in hearing gerbils in both approaches. We did not detect ChR expression in the central nervous system using microcatheter administration. Comparing optogenetic auditory brainstem responses of gerbils with and without hair cell transduction confirmed that SGNs were the primary site of optogenetic stimulation of the pathway. Conclusions: The AAV.PHP-S microcatheter administration via the round window with pressure relief at the round window is a reliable approach to optogenetically modify the SGNs in order to restore hearing with future optical cochlear implants.
Keywords: cochlear optogenetic stimulation; microcatheter-based administration; optical cochlear implants; spiral ganglion neurons.; viral vector delivery.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- WHO. Deafness and hearing loss [Internet]. WHO; 2021 Jan [cited 10 July 2022]. Available at: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss .
-
- Lv J, Wang H, Cheng X. et al. AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial. The Lancet. 2024;403:2317–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

