EphA2-targeted alpha-particle theranostics for enhancing PDAC treatment
- PMID: 40225586
- PMCID: PMC11984392
- DOI: 10.7150/thno.106948
EphA2-targeted alpha-particle theranostics for enhancing PDAC treatment
Abstract
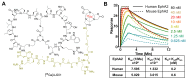
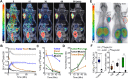
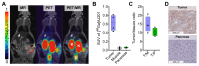
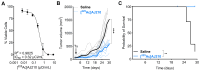
Background: Pancreatic ductal adenocarcinoma (PDAC) presents a formidable challenge in oncology due to its aggressive nature and resistance to therapy. Current treatments, including surgery, chemotherapy, and radiotherapy, have limited success in improving patient outcomes. This study addresses the urgent need for novel radiotheranostic strategies for PDAC by investigating EphA2 as a potential target. Methods and Results: Analysis of genomic data from the Cancer Cell Line Encyclopedia (CCLE) and The Cancer Genome Atlas (TCGA) revealed elevated EphA2 expression in PDAC, confirmed by immunohistochemical staining of tumor tissue microarrays (TMAs). Further analysis showed variable EphA2 expression across PDAC cell lines, with surface receptor density not always correlating with mRNA levels. A low molecular weight peptide was developed and labeled with gallium-68 for PET imaging. In vitro studies demonstrated specific binding to EphA2-expressing PDAC cells with rapid internalization. In vivo PET imaging in subcutaneous and orthotopic PDAC models confirmed high tumor uptake and minimal off-target binding, confirming EphA2 as a valid imaging target. For molecular radiotherapy, a DOTA-conjugated peptide was labeled with the alpha-particle emitter, actinium-225. In vitro studies revealed dose-dependent cytotoxicity in PDAC cells, with an IC50 of 0.32 µCi/mL. In a tumor model, treatment with Ac-225 labeled peptide significantly inhibited tumor growth compared to controls, with mild adverse effects. Conclusion: These results establish EphA2 as a promising radiotheranostic target in PDAC, with potential for both non-invasive imaging and targeted radiotherapy. Given the potential, further optimization of EphA2-targeted agents are warranted to advance personalized treatment strategies for PDAC patients.
Keywords: Alpha-particle therapy; Gallium-68; Imaging; PET; Pancreatic cancer; Peptide radiopharmaceuticals; Radiotherapy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV. et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. - PubMed
-
- Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW. et al. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res. 2017;23:4617–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

