Acupuncture alleviates CSDS-induced depressive-like behaviors by modulating synaptic plasticity in vCA1
- PMID: 40225589
- PMCID: PMC11984413
- DOI: 10.7150/thno.106751
Acupuncture alleviates CSDS-induced depressive-like behaviors by modulating synaptic plasticity in vCA1
Abstract
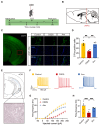
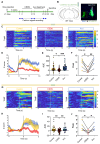
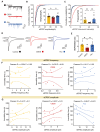
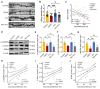
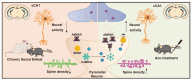
Acupuncture (Acu) has been clinically validated as an effective treatment for depression. However, the underlying mechanism of Acu treatment's antidepressant effect remains unclear. Methods: We investigate the antidepressant effects of Acu treatment at the LR3 point in mice subjected to chronic social defeat stress (CSDS). GCaMP6m-based fiber-optic photometry was employed in the ventral CA1 (vCA1) regions for the first time to monitor Ca2+ transients in vivo during behavioral testing. Electrophysiological recordings were used to detect the activity and synaptic function of pyramidal neurons. Golgi staining was performed to measure the density of dendritic spines in the vCA1. Western blot analysis was conducted to quantify the expression levels of phosphorylated CaMKIIα, AMPA receptor protein (GluA1, GluA2), and brain-derived neurotrophic factor (BDNF) in the hippocampus. Results: Our findings indicated that Acu treatment significantly alleviated emotional deficits and restored the activity of pyramidal neurons, which were suppressed by CSDS. Acu treatment also reversed the decrease in spontaneous excitatory postsynaptic currents (sEPSCs), thereby enhancing glutamatergic transmission. Moreover, Acu treatment improved synaptic plasticity, as evidenced by increased dendritic spine density and restored expression levels of phosphorylated CaMKIIα, GluA1, GluA2 and BDNF. Conclusion: Collectively, these findings suggest that Acu treatment alleviates depressive-like behaviors induced by CSDS and enhances synaptic function in the vCA1 region, potentially through mechanisms involving increased AMPAR trafficking and BDNF expression.
Keywords: LR3; acupuncture; depression; fiber photometry; synaptic plasticity.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Sampogna G, Toni C, Catapano P, Rocca BD, Di Vincenzo M, Luciano M. et al. New trends in personalized treatment of depression. Curr Opin Psychiatry. 2024;37:3–8. - PubMed
-
- Barcelos-Ferreira R, Izbicki R, Steffens DC, Bottino CM. Depressive morbidity and gender in community-dwelling Brazilian elderly: systematic review and meta-analysis. Int Psychogeriatr. 2010;22:712–26. - PubMed
-
- Torjesen I. Adding acupuncture or counselling to usual care hastens improvement in persistent depression. BMJ. 2013;347:f5789. - PubMed
-
- Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet (London, England) 2023;401:141–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

