Economic Impact of Elranatamab for Treatment of Patients with Relapsed or Refractory Multiple Myeloma
- PMID: 40225701
- PMCID: PMC11992987
- DOI: 10.2147/CEOR.S501404
Economic Impact of Elranatamab for Treatment of Patients with Relapsed or Refractory Multiple Myeloma
Abstract
Purpose: To estimate the budget impact of adding elranatamab to the US formulary to treat adults with RRMM who have received ≥4 prior lines of therapy including a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 monoclonal antibody, and to assess the total cost of care and cost per month of progression-free survival (PFS) between elranatamab and available treatments.
Methods: An economic model was developed to assess the budget impact of elranatamab in a one-million-member US commercial and Medicare health plan. Epidemiology data was obtained from the SEER database and a large US real-world study. Key clinical inputs included treatment duration, PFS, overall survival, and adverse events (AEs). Costs associated with drug acquisition monitoring, medical resource use (specifically hospitalization and physician visits), and AEs were incorporated. Model inputs were sourced from clinical trial data, US government databases, and published literature. Total budget impact and per member per month (PMPM) were assessed. One-way sensitivity analyses (OWSA) were conducted to assess model input uncertainty. Total cost of care and cost per month of PFS were also assessed.
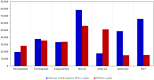
Results: An estimated 14 (commercial) and 60 (Medicare) RRMM patients per year would be eligible for treatment. Adding elranatamab resulted in a total budget impact of $553,607 ($0.05 PMPM) in commercial and $2,351,515 ($0.20 PMPM) in Medicare over three years. OWSA indicated results were most sensitive for elranatamab drug costs and relative dose intensity. Total cost of care per month of median PFS over one year was $19,642 with elranatamab, talquetamab ($33,391), teclistamab ($37,791), selinexor plus dexamethasone ($48,784), physician's choice of treatment ($65,886), idecabtagene vicleucel ($78,361), and ciltacabtagene autoleucel ($17,640).
Conclusion: Elranatamab for RRMM is projected to result in a minimal to small budget impact over 3 years and good economic value with lower cost of care per month of PFS compared with other available RRMM treatments except for ciltacabtagene autoleucel.
Keywords: US commercial payer; budget impact model; cost of care; medicare; multiple myeloma; triple-class exposed; triple-class refractory.
© 2025 Shah et al.
Conflict of interest statement
BS: participated on an advisory board for Pfizer. YL, YH, and IM: employed by Cytel, which received funding from Pfizer in connection with the development of this manuscript. LB: participated on an advisory board for Pfizer. RS, DH, JH, and PH: employed by Pfizer and earn stock and/or stock options. AS: formerly employed by Pfizer and earns stock and/or stock options. The authors report no other conflicts of interest in this work.
Figures


References
-
- National Cancer Institute. Surveillance, epidemiology, and end results program: cancer stat facts: myeloma. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed December, 2023.
-
- Multiple myeloma research foundation. Understanding multiple myeloma. Available from: https://themmrf.org/multiple-myeloma/. Accessed December 2023.
-
- American Cancer Society. Multiple myeloma. Available from: https://www.cancer.org/cancer/types/multiple-myeloma.html. Accessed December 2023.
LinkOut - more resources
Full Text Sources
Research Materials

