Immune Checkpoint Inhibitor-Mediated Aseptic Meningitis and Hypophysitis
- PMID: 40225817
- PMCID: PMC11991839
- DOI: 10.1155/crom/3517328
Immune Checkpoint Inhibitor-Mediated Aseptic Meningitis and Hypophysitis
Abstract
Immune checkpoint inhibitors have revolutionized cancer treatment, yet their use is associated with unique and sometimes unpredictable immune-related adverse events. We present a case of a 67-year-old female with renal cell cancer treated with ipilimumab and nivolumab who developed aseptic meningitis and hypophysitis. This case highlights the challenges in managing immune-related adverse events and underscores the need for vigilance in monitoring patients receiving ICIs.
Keywords: immunotherapy; ipilimumab; nivolumab; oncology; renal cell carcinoma.
Copyright © 2025 Pavel Bleik et al. Case Reports in Oncological Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


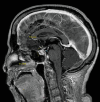
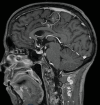
Similar articles
-
Late-Onset Hypophysitis Induced by Nivolumab in Advanced Esophageal Squamous Cell Carcinoma.Cureus. 2025 Apr 6;17(4):e81806. doi: 10.7759/cureus.81806. eCollection 2025 Apr. Cureus. 2025. PMID: 40330397 Free PMC article.
-
Exploring a Rarity: Incidence of and Therapeutic Approaches for Neurological Complications and Hypophysitis in Cancer Patients on Immune Checkpoint Inhibitors-A Single-Center Study.Curr Oncol. 2023 Dec 18;30(12):10509-10518. doi: 10.3390/curroncol30120766. Curr Oncol. 2023. PMID: 38132400 Free PMC article.
-
Immune checkpoint inhibitor-induced aseptic meningitis and encephalitis: a case-series and narrative review.Ther Adv Drug Saf. 2021 Mar 29;12:20420986211004745. doi: 10.1177/20420986211004745. eCollection 2021. Ther Adv Drug Saf. 2021. PMID: 33854755 Free PMC article.
-
Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: a case report and review of the literature.J Med Case Rep. 2019 Mar 26;13(1):88. doi: 10.1186/s13256-019-2002-2. J Med Case Rep. 2019. PMID: 30909965 Free PMC article. Review.
-
Perimyocarditis Associated with Immune Checkpoint Inhibitors: A Case Report and Review of the Literature.Medicina (Kaunas). 2024 Jan 28;60(2):224. doi: 10.3390/medicina60020224. Medicina (Kaunas). 2024. PMID: 38399513 Free PMC article. Review.
References
-
- Hartmann A., Paparoupa M., Volkmer B. G., Rompel R., Wittig A., Schuppert F. Autoimmune hypophysitis secondary to therapy with immune checkpoint inhibitors: four cases describing the clinical heterogeneity of central endocrine dysfunction. Journal of Oncology Pharmacy Practice . 2020;26(7):1774–1779. doi: 10.1177/1078155220910202. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

