Frequency, Antimicrobial Susceptibility, and Molecular Characterization of Carbapenem-Resistant Enterobacterales Stratified by United States Census Divisions: Results From the INFORM Program (2018-2022)
- PMID: 40225828
- PMCID: PMC11986335
- DOI: 10.1093/ofid/ofaf005
Frequency, Antimicrobial Susceptibility, and Molecular Characterization of Carbapenem-Resistant Enterobacterales Stratified by United States Census Divisions: Results From the INFORM Program (2018-2022)
Abstract
Background: Recently approved β-lactamase inhibitor combinations, such as ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam, have demonstrated a broad spectrum of activity against carbapenem-resistant Enterobacterales (CRE) from US hospitals, but resistance may emerge with the increasing use of these compounds. Aztreonam-avibactam was recently approved in Europe and it is under clinical development in the United States. We evaluated the activity of aztreonam-avibactam and comparators against CREs from US hospitals.
Methods: A total of 45 497 Enterobacterales isolates were consecutively collected from 79 US medical centers (36 states) and susceptibility tested by broth microdilution. Aztreonam-avibactam was tested with avibactam at a fixed 4 mg/L and a susceptible breakpoint of ≤4 mg/L was applied for comparison. CRE isolates were screened for carbapenemase by whole-genome sequencing.
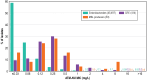
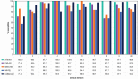
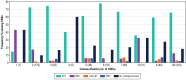
Results: Aztreonam-avibactam inhibited >99.9% of Enterobacterales at ≤4 mg/L. CRE frequencies varied from 0.2% (New England) to 2.4% (Middle Atlantic). Aztreonam-avibactam was active (minimum inhibitory concentration ≤4 mg/L) against 98.6% (408/414) of CREs overall, whereas susceptibility to ceftazidime-avibactam and meropenem-vaborbactam were lowest in the Mountain division (67.7% and 74.2%, respectively) and highest (100.0%) in West North Central. Klebsiella pneumoniae carbapenemase was the most common carbapenemase (65.5% of CREs), followed by New Delhi MBL (10.6%) and oxacillinase-48-like (2.7%). The occurrence of Klebsiella pneumoniae carbapenemase among CREs varied from 14.3% (New England) to 77.8% (East South Central), whereas the frequency of MBLs ranged from ≤3.0% (4 divisions) to 19.4% in Mountain and 42.9% in New England.
Conclusions: Aztreonam-avibactam showed potent activity against CRE, including MBL producers. Resistance to ceftazidime-avibactam and meropenem-vaborbactam was observed among CRE because of increasing occurrence of MBL-producing isolates.
Keywords: CRE; KPC; NDM; OXA-48; metallo-beta-lactamase.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. None of the authors has a conflict of interest. Element Iowa City (JMI Laboratories) was contracted to perform services in 2022–2024 for AimMax Therapeutics, Amicrobe, Inc., Appili Therapeutics, Armata Pharmaceuticals, Astellas Pharma, Inc., Basilea Pharmaceutica AG, Biosergen AB, Bugworks, Cerba Research NV, Cidara Therapeutics, Cipla USA Inc., ContraFect Corporation, CorMedix Inc., Crestone, Inc., Curza Global, LLC, Diamond V, Discuva Ltd., Entasis Therapeutics, Enveda Biosciences, Evopoint Biosciences, Fedora Pharmaceuticals, Fox Chase Chemical Diversity Center, Genentech, Gilead Sciences, Inc., GSK plc, Iterum Therapeutics plc, Janssen Biopharma, Johnson & Johnson, Kaleido Biosciences, LifeMine Therapeutics, Medpace, Inc, Lysovant Sciences, Inc, Meiji Seika Pharma, Melinta Therapeutics, Menarini Group, Merck & Co., MicuRx Pharmaceutical Inc., Mundipharma International Ltd., Mutabilis, Nabriva Therapeutics, National Cancer Institute, National Institutes of Health, Ohio State University, Omnix Medical Ltd., Paratek Pharmaceuticals, Pfizer, PolyPid Ltd., PPD, Prokaryotics, Inc., Pulmocide Ltd, Qpex Biopharma, Revagenix, Roche Holding AG, Roivant Sciences, Scynexis, Inc., Shionogi & Co., Ltd., Sinovent Pharmaceuticals, Inc., Spero Therapeutics, Sumitovant Biopharma, Inc., TenNor Therapeutics, U.S. Food and Drug Administration, VenatoRx Pharmaceuticals, Washington University, Watershed Medical, LLC, Wockhardt, and Zoetis, Inc.
Figures
Similar articles
-
Antimicrobial susceptibility of enterobacterales causing bloodstream infection in United States medical centres: comparison of aztreonam-avibactam with beta-lactams active against carbapenem-resistant enterobacterales.BMC Infect Dis. 2024 Nov 5;24(1):1242. doi: 10.1186/s12879-024-10133-5. BMC Infect Dis. 2024. PMID: 39501203 Free PMC article.
-
Activity of aztreonam-avibactam against Enterobacterales resistant to recently approved beta-lactamase inhibitor combinations collected in Europe, Latin America, and the Asia-Pacific Region (2020-2022).Int J Antimicrob Agents. 2024 Apr;63(4):107113. doi: 10.1016/j.ijantimicag.2024.107113. Epub 2024 Feb 12. Int J Antimicrob Agents. 2024. PMID: 38354826
-
Activity of Aztreonam-avibactam and other β-lactamase inhibitor combinations against Gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2020-2022).BMC Pulm Med. 2025 Jan 24;25(1):38. doi: 10.1186/s12890-025-03500-8. BMC Pulm Med. 2025. PMID: 39856702 Free PMC article.
-
Review of Ceftazidime-Avibactam, Meropenem-Vaborbactam, and Imipenem/Cilastatin-Relebactam to Target Klebsiella pneumoniae Carbapenemase-Producing Enterobacterales.J Pharm Technol. 2020 Oct;36(5):202-210. doi: 10.1177/8755122520934726. Epub 2020 Jun 17. J Pharm Technol. 2020. PMID: 34752560 Free PMC article. Review.
-
New β-Lactam-β-Lactamase Inhibitor Combinations.Clin Microbiol Rev. 2020 Nov 11;34(1):e00115-20. doi: 10.1128/CMR.00115-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33177185 Free PMC article. Review.
Cited by
-
Activity of β-Lactamase Inhibitor Combinations Against Enterobacterales Isolated from Patients with Intra-Abdominal Infection from United States Medical Centres (2019-2023).Antibiotics (Basel). 2025 May 27;14(6):544. doi: 10.3390/antibiotics14060544. Antibiotics (Basel). 2025. PMID: 40558134 Free PMC article.
References
-
- Paniagua-Garcia M, Bravo-Ferrer JM, Perez-Galera S, et al. Attributable mortality of infections caused by carbapenem-resistant Enterobacterales: results from a prospective, multinational case-control-control matched cohorts study (EURECA). Clin Microbiol Infect 2024; 30:223–30. - PubMed
-
- Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant Gram-negative infections. Clin Infect Dis 2024; ciae403 [in press]. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

