Single and Multiple Doses of Seladelpar Decrease Diurnal Markers of Bile Acid Synthesis in Mice
- PMID: 40225907
- PMCID: PMC11991775
- DOI: 10.1155/ppar/5423221
Single and Multiple Doses of Seladelpar Decrease Diurnal Markers of Bile Acid Synthesis in Mice
Abstract
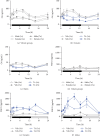
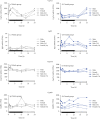
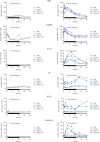
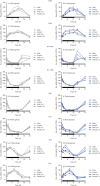
Peroxisome proliferator-activated receptors (PPARs) modulate bile metabolism and are important therapeutic options in cholestatic diseases. This study was aimed at understanding the effects of single and multiple doses of seladelpar, a PPARδ (peroxisome proliferator-activated receptor delta) agonist, on plasma C4 (a freely diffusible metabolite accepted as a proxy for de novo bile acid biosynthesis), Fibroblast Growth Factor 21 (Fgf21), and gene expression changes in the liver of male and female mice. C57BL/6 mice were treated with seladelpar 10 mg/kg/day or vehicle through oral gavage before lights out on Day 1 (single dose) or from Day 1 to Day 7 (multiple doses). Liver samples were obtained at 0, 1, 2, 4, 8, 12, 16, and 24 h postdosing, and plasma C4 and Fgf21 levels were measured. In vehicle-treated mice, C4 levels were higher in the dark cycle compared to the light cycle, with higher levels in females than in males. Plasma Fgf21 did not vary substantially over the dark-light cycle or show a sex-specific expression pattern. Seladelpar treatment significantly reduced plasma C4 and increased Fgf21 levels in both sexes, which coincided with a decrease in cholesterol 7α-hydroxylase mRNA and an increase in Fgf21 mRNA in the livers. Untargeted RNA sequencing revealed a strong correlation between the genes differentially expressed after single- and multiple-dose seladelpar treatment. PPAR-responsive genes, including pyruvate dehydrogenase kinase 4, acyl-CoA thioesterase 2, and angiopoietin-like 4, were upregulated. No changes in nuclear receptors, clock genes, and sex-specific genes were observed. Overall, these results are consistent with a model where seladelpar treatment reduces bile acid synthesis by upregulating Fgf21 and modulating other PPAR-responsive genes.
Keywords: PPAR; PPAR delta; bile metabolism; circadian rhythm; seladelpar.
Copyright © 2025 Edward E. Cable et al. PPAR Research published by John Wiley & Sons Ltd.
Conflict of interest statement
Edward E. Cable, Jeffrey W. Stebbins, Jeff D. Johnson, Yun-Jung Choi, Jiangao Song, and Charles A. McWherter are employees of CymaBay Therapeutics Inc. Sole Gatto and Matthew Onorato are employees of Monoceros Biosystems LLC.
Figures


Similar articles
-
Selective PPARδ agonist seladelpar suppresses bile acid synthesis by reducing hepatocyte CYP7A1 via the fibroblast growth factor 21 signaling pathway.J Biol Chem. 2022 Jul;298(7):102056. doi: 10.1016/j.jbc.2022.102056. Epub 2022 May 20. J Biol Chem. 2022. PMID: 35605662 Free PMC article.
-
Seladelpar combined with complementary therapies improves fibrosis, inflammation, and liver injury in a mouse model of nonalcoholic steatohepatitis.Am J Physiol Gastrointest Liver Physiol. 2024 Feb 1;326(2):G120-G132. doi: 10.1152/ajpgi.00158.2023. Epub 2023 Nov 28. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38014444 Free PMC article.
-
The selective PPAR-delta agonist seladelpar reduces ethanol-induced liver disease by restoring gut barrier function and bile acid homeostasis in mice.Transl Res. 2021 Jan;227:1-14. doi: 10.1016/j.trsl.2020.06.006. Epub 2020 Jun 15. Transl Res. 2021. PMID: 32553670 Free PMC article.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
-
PPAR agonists for the treatment of cholestatic liver diseases: Over a decade of clinical progress.Hepatol Commun. 2024 Dec 20;9(1):e0612. doi: 10.1097/HC9.0000000000000612. eCollection 2025 Jan 1. Hepatol Commun. 2024. PMID: 39699308 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

