Rhomboid proteases: key players at the cell surface within haloarchaea
- PMID: 40226099
- PMCID: PMC11985538
- DOI: 10.3389/fmicb.2025.1547649
Rhomboid proteases: key players at the cell surface within haloarchaea
Abstract
Introduction: Rhomboid proteases are intramembrane serine proteases that play a key role in regulating membrane proteins across all domains of life. However, their function in archaea remains poorly understood. The model halophilic archaeon Haloferax volcanii encodes two rhomboid homologs, rho1 (HVO_1474) and rho2 (HVO_0727). Previous studies indicated that the deletion of rho2 resulted in mild alterations in motility, adhesion, biofilm formation, and cell morphology, suggesting potential functional compensation by rho1.
Materials and methods: To investigate the role of these proteases, we generated single (Δrho1) and double (Δrho1 Δrho2) deletion mutants. Phenotypic characterization included viability assays, motility tests, adhesion and biofilm formation studies, as well as morphological analysis using microscopy. Functional overlap between rho1 and rho2 was evaluated through genetic complementation/overexpression experiments in which each gene was expressed in trans in the mutant backgrounds.
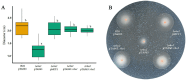
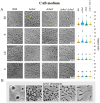
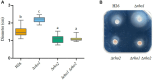
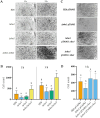
Results: Both Δrho1 and Δrho1 Δrho2 mutants were viable, indicating that these genes are not essential in H. volcanii. The Δrho1 mutant exhibited increased motility, enhanced biofilm formation, reduced adhesion to glass surfaces, and significant morphological alterations, particularly in trace element-deficient conditions. The double mutant (Δrho1 Δrho2) showed increased adhesion to surfaces, mild motility reduction, and fewer morphological abnormalities compared to Δrho1. Complementation assays revealed that both rho1 and rho2 could restore motility in Δrho2 and adhesion in Δrho1. However, only rho1 was able to complement the morphological defects, suggesting a degree of functional divergence between these homologs.
Discussion: This work highlights the role of rhomboid proteases in regulating critical cell surface processes in H. volcanii, including biofilm formation, surface adhesion, and cell shape determination. The ability of rho1 and rho2 to compensate for each other in certain functions while maintaining distinct roles underscores a complex regulatory interplay. Future research will focus on identifying natural substrates and elucidating the molecular mechanisms underlying rhomboid protease function in haloarchaea.
Keywords: Haloferax volcanii; archaeal morphology; haloarchaea; intramembrane proteases; rhomboid protease.
Copyright © 2025 Costa, Cerletti, Paggi, Frecha, Zoratti, Latorre, De Castro and Giménez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abdul-Halim M. F., Karch K. R., Zhou Y., Haft D. H., Garcia B. A., Pohlschroder M. (2015). Permuting the PGF signature motif blocks both Archaeosortase-dependent C-terminal cleavage and Prenyl lipid attachment for the Haloferax volcanii S-layer glycoprotein. J. Bacteriol. 198, 808–815. doi: 10.1128/JB.00849-15, PMID: - DOI - PMC - PubMed
-
- Allers T. (2009). The Halohandbook: Protocols for haloarchaeal genetics. Melbourne, VI, Australia: Haloarchaeal Genetics Laboratory.
LinkOut - more resources
Full Text Sources

