A Lyme disease mRNA vaccine targeting Borrelia burgdorferi OspA induces strong immune responses and prevents transmission in mice
- PMID: 40226328
- PMCID: PMC11986965
- DOI: 10.1016/j.omtn.2025.102514
A Lyme disease mRNA vaccine targeting Borrelia burgdorferi OspA induces strong immune responses and prevents transmission in mice
Abstract
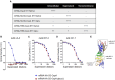
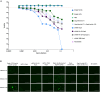
Lyme borreliosis (LB), caused by Borrelia burgdorferi sensu lato, is one of the most common tick-borne diseases in the northern hemisphere. Given its increasing global incidence, LB remains a major public health concern and the development of an effective vaccine is recognized as a key component of the overall disease prevention strategy. Here, we present results obtained with newly developed lipid nanoparticle-encapsulated mRNA vaccine candidates encoding the outer surface protein A (OspA) of B. burgdorferi sensu stricto (Bbss) serotype 1 (mRNA-OspA) with or without a secretion signal (SS) or a transmembrane domain. We evaluated the immunogenicity and protective efficacy of the mRNA-OspA vaccine candidates in a tick-fed mouse challenge model compared with an adjuvanted OspA protein subunit vaccine and the licensed canine vaccine Recombitek Lyme. At the doses tested, the mRNA-OspA vaccines induced significantly higher OspA-specific immunoglobulin G titers than the protein-based vaccines, as well as functional antibodies measured by serum bactericidal assay against Bbss strain B31. Complete protection against transmission was observed in the group immunized with the mRNA-OspA without SS. Overall, these data demonstrate that an mRNA-OspA vaccine can be effective against LB infection and could be used in the future for the prevention of Lyme disease.
Keywords: Borrelia burgdorferi; Lyme borreliosis; MT: Oligonucleotides: Therapies and Applications; OspA; immunogenicity; mRNA vaccines; outer surface protein A; tick-borne pathogens; ticks; vector-borne disease control.
© 2025 The Authors.
Conflict of interest statement
Authors have declared the following interests: V.P., C.C., Y.G.C., S.M., and C.P. are Sanofi employees and may own Sanofi shares.
Figures




References
-
- Wilske B., Preac-Mursic V., Göbel U.B., Graf B., Jauris S., Soutschek E., Schwab E., Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J. Clin. Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

