Engineered Decellularized Tendon Matrix Putty Preserves Native Tendon Bioactivity to Promote Cell Proliferation and Enthesis Repair
- PMID: 40226422
- PMCID: PMC11918894
- DOI: 10.1155/2023/4665795
Engineered Decellularized Tendon Matrix Putty Preserves Native Tendon Bioactivity to Promote Cell Proliferation and Enthesis Repair
Abstract
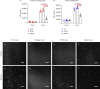
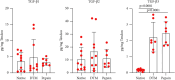
Rotator cuff tears are a common soft tissue injury that can significantly decrease function of the shoulder and cause severe pain. Despite progress in surgical technique, rotator cuff repairs (RCRs) do not always heal efficiently. Many failures occur at the bone-tendon interface as a result of poor healing capacity of the tendon and failure to regenerate the native histological anatomy of the enthesis. While allografts are commercially available, clinical use is limited as they do not stimulate tissue regeneration and are associated with a structural failure of up to 40% in re-tear cases. Novel tissue engineering strategies are being developed with promise, but most involve addition of cells and/or growth factors which extends the timeline for clinical translation. Thus, there exists a significant unmet clinical need for easily translatable surgical augmentation approaches that can improve healing in RCR. Here we describe the development of a decellularized tendon matrix (DTM) putty that preserves native tendon bioactivity using a novel processing technique. In vitro, DTM promoted proliferation of tenocytes and adipose-derived stem cells with an increase in expression-specific transcription factors seen during enthesis development, Scleraxis and Sox9. When placed in a rabbit model of a chronic rotator cuff tear, DTM improved histological tissue repair by promoting calcification at the bone-tendon interface more similar to the normal fibrocartilaginous enthesis. Taken together, these data indicate that the engineered DTM putty retains a pro-regenerative bioactivity that presents a promising translational strategy for improving healing at the enthesis.
Copyright © 2023 Anna-Laura Nelson et al.
Conflict of interest statement
Kelsey O'Hara, Dr. Phillip Nolte, Dr. Naomasa Fukase, Dr. Yoichi Murata, Dr. Anna Tross, and Dr. Johnny Huard have no conflicts of interest to disclose. Dr. Millett discloses royalties from Arthrex Inc. and Springer. Dr. Millett also discloses a paid consultant position and research support from Arthrex Inc. and holds stock with VuMedi. Dr. Chelsea S. Bahney discloses an unpaid position on the leadership for Orthopaedic Research Society (ORS), Tissue Engineering and Regenerative Medicine International Society (TERMIS), the Orthopaedic Trauma Association (OTA), and the AO Foundation CMF R&D Committee. CSB also discloses IP royalties from Iota Biosciences Inc. for US Patent 041263 and an Associate Editor role for the Journal of Tissue Engineering and Regenerative Medicine (JTERM). Drs. Millett, Bahney, Bernholt, and Anna Laura Nelson also disclose inventorship on PCT/US2021/038165 (6/20/2021)/WO2021258034A1 (12/23/2021) Decellularized Tendon Matrix (DTM) Methods and uses thereof. Anna Laura Nelson, Dr. Johnny Huard, and Dr. Chelsea Bahney are all paid employees of the non-profit Steadman Philippon Research Institute (SPRI). SPRI exercises special care to identify any financial interests or relationships related to research conducted here. During the past calendar year, SPRI has received grant funding or in-kind donations from Arthrex, Canon, DJO, Icarus Medical, Medtronic, Ossur, Smith + Nephew, SubioMed, Stryker, and Wright Medical. These funding sources provided no support for the work presented in this manuscript.
Figures





References
-
- ltd R., (n.d.) M. Sports medicine market report suite-united states-2020–2026-medsuite . Sports Medicine Market Report Suite; 2019. https://www.researchandmarkets.com/reports/4876618/sports-medicine-marke... .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

