Applied Electric Fields Polarize Initiation and Growth of Endothelial Sprouts
- PMID: 40226427
- PMCID: PMC11919209
- DOI: 10.1155/2023/6331148
Applied Electric Fields Polarize Initiation and Growth of Endothelial Sprouts
Abstract
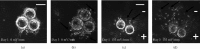
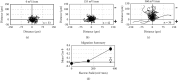
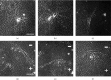
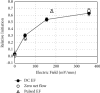
Therapeutic electric fields (EFs) are applied to the epidermis to accelerate the healing of chronic epidermal wounds and promote skin transplantation. While research has emphasized understanding the role of EFs in polarizing the migration of superficial epidermal cells, there are no reports describing the effect of EFs on polarization of the underlying vasculature. We explored the effects of EFs on the growth of endothelial sprouts, precursors to functional blood vessels. We discovered that DC EFs of the same magnitude near wounded epidermis polarize initiation, growth, and turning of endothelial sprouts toward the anode. While EFs polarize sprouts, they do not change the frequency of primary sprout or branch formation. Unidirectional electrical pulses also polarize sprouts based on their time-averaged EF magnitude. Sprout polarization occurs antiparallel to the direction of electrically driven water flow (electro-osmosis) and is consistent with the direction of sprout polarization induced by pressure-driven flow. These results support the role of EFs in controlling the direction of neovascularization during the healing of soft tissues and tissue engineering.
Copyright © 2023 Anyesha Sarkar et al.
Conflict of interest statement
The authors have no relevant financial or nonfinancial conflicts of interest to disclose.
Figures





Similar articles
-
Directing migration of endothelial progenitor cells with applied DC electric fields.Stem Cell Res. 2012 Jan;8(1):38-48. doi: 10.1016/j.scr.2011.08.001. Epub 2011 Aug 16. Stem Cell Res. 2012. PMID: 22099019 Free PMC article.
-
Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients.Lab Chip. 2010 Nov 21;10(22):3061-8. doi: 10.1039/c005069e. Epub 2010 Sep 1. Lab Chip. 2010. PMID: 20820484
-
Electric field-induced suppression of PTEN drives epithelial-to-mesenchymal transition via mTORC1 activation.J Dermatol Sci. 2017 Feb;85(2):96-105. doi: 10.1016/j.jdermsci.2016.11.007. Epub 2016 Nov 18. J Dermatol Sci. 2017. PMID: 27919618
-
Extracellular electrical fields direct wound healing and regeneration.Biol Bull. 2011 Aug;221(1):79-92. doi: 10.1086/BBLv221n1p79. Biol Bull. 2011. PMID: 21876112 Review.
-
Angiogenesis in wound healing.J Investig Dermatol Symp Proc. 2000 Dec;5(1):40-6. doi: 10.1046/j.1087-0024.2000.00014.x. J Investig Dermatol Symp Proc. 2000. PMID: 11147674 Review.
Cited by
-
Electroactive Electrospun Nanofibrous Scaffolds: Innovative Approaches for Improved Skin Wound Healing.Adv Sci (Weinh). 2025 May;12(18):e2416267. doi: 10.1002/advs.202416267. Epub 2025 Apr 7. Adv Sci (Weinh). 2025. PMID: 40190057 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

