Osilodrostat Treatment of Cushing Syndrome in Real-World Clinical Practice: Findings From the ILLUSTRATE study
- PMID: 40226519
- PMCID: PMC11986586
- DOI: 10.1210/jendso/bvaf046
Osilodrostat Treatment of Cushing Syndrome in Real-World Clinical Practice: Findings From the ILLUSTRATE study
Abstract
Context: In clinical trials, osilodrostat (11β-hydroxylase inhibitor) effectively reduced cortisol levels in patients with endogenous Cushing syndrome (CS).
Objectives: A real-world study (ILLUSTRATE) was conducted evaluating osilodrostat use in patients with various etiologies of CS in the United States.
Methods: A retrospective chart-review study was conducted of adults with CS treated with osilodrostat between May 1, 2020, and October 29, 2021.
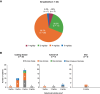
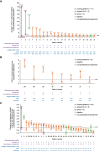
Results: A total of 42 patients (Cushing disease, n = 34; CS due to adrenal adenoma, n = 5; ectopic adrenocorticotropin syndrome [EAS], n = 3) were included. Starting doses were 2 mg twice daily in 27/42 patients (64.3%), maintenance doses were 2 mg twice daily in 6 of 9 patients (66.7%) attaining them. During osilodrostat treatment, urinary free cortisol (UFC) decreased below the upper limit of normal (ULN) in 14 of 20 patients (70.0%) with pretreatment UFC greater than the ULN. Osilodrostat response was observed across a range of doses (2-20 mg/day). In Cushing disease, median UFC and late-night salivary cortisol decreased from 3.03 and 2.39 × ULN, respectively, to 0.71 and 1.13 × ULN at last assessment in those with available data (n = 17 and 8, respectively). UFC decreased in all patients with adrenal CS or EAS with available data (n = 2 each). There were no unexpected safety signals; the most common adverse events (incidence ≥20%) were fatigue, nausea, and lower-extremity edema. Glucocorticoid withdrawal syndrome and/or adrenal insufficiency were reported in 12 of 42 patients (28.6%) after osilodrostat initiation, resulting in treatment discontinuation in 4.
Conclusion: In routine practice with dosing individualized according to clinical condition, response, and tolerability, osilodrostat was effective and well tolerated regardless of CS etiology and severity.
Keywords: Cushing disease; adrenal Cushing syndrome; ectopic adrenocorticotropin syndrome; osilodrostat; real world; retrospective.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Fleseriu M, Varlamov EV, Hinojosa-Amaya JM, Langlois F, Melmed S. An individualized approach to the management of Cushing disease. Nat Rev Endocrinol. 2023;19(10):581‐599. - PubMed
-
- Gadelha M, Gatto F, Wildemberg LE, Fleseriu M. Cushing's syndrome. Lancet. 2023;402(10418):2237‐2252. - PubMed
-
- Braun LT, Vogel F, Reincke M. Long-term morbidity and mortality in patients with Cushing's syndrome. J Neuroendocrinol. 2022;34(8):e13113. - PubMed
-
- Reincke M, Fleseriu M. Cushing syndrome: a review. JAMA. 2023;330(2):170‐181. - PubMed
LinkOut - more resources
Full Text Sources
