OnabotulinumtoxinA in Resistant Depression: A Randomized Trial Comparing Two Facial Injection Sites (OnaDEP Study)
- PMID: 40226647
- PMCID: PMC11918888
- DOI: 10.1155/2024/1177925
OnabotulinumtoxinA in Resistant Depression: A Randomized Trial Comparing Two Facial Injection Sites (OnaDEP Study)
Abstract
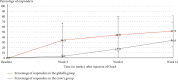
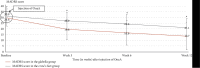
Background: OnabotulinumtoxinA (OnaA) injection in glabella area appears to be a promising treatment for major depression. However, one major concern of placebo-controlled studies on botulinum toxin injections is to ensure adequate blinding. Patients and Methods: In this context, all subjects of this trial received the active product (OnaA). After randomization, 58 patients with resistant major depressive disorder (MDD) received OnaA either in the glabella area (N = 29) or in the crow's feet area (N = 29). Subjects were blinded to the supposedly effective area against resistant depression and the examiner was not aware of the injected area. The primary outcome measure was the proportion of responders (50% or greater decrease in MADRS [Montgomery and Asberg Depression Rating Scale] score from baseline) in glabella group versus crow's feet group at week 6 after the OnaA injection. Results: The number of responders was significantly higher in the glabella group than in the crow's feet group with 13 responders out of 29 patients (44.8%) in the glabella group and five out of 28 patients (17.9%) in the crow's feet group (p=0.029). The rate of psychomotor agitation as measured by item 9 of the Hamilton Depression Rating Scale (HAM-D), associated with a shorter span of psychiatric disorder, was a potent positive predictive factor of positive response to treatment. Conclusion: We conclude that OnaA injected in the glabella muscles is an effective and well-tolerated treatment for MDD. We suggest that patients with a high score at item 9 of the HAM-D might be a subgroup of best responders. We assume that OnaA may act as a modulator of the activity of the primary sensorimotor cortex and then of the amygdala. Trial Registration: ClinicalTrials.gov identifier: NCT03484754.
Keywords: agitation; botulinum toxin A; depression; melancholic omega; neuromodulation: amygdala.
Copyright © 2024 Caroline Ceolato-Martin et al.
Conflict of interest statement
Ranoux Danièle is a consultant for Merz and Ipsen. All other authors declare no conflicts of interest.
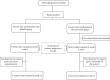
Figures
References
-
- Institute for Health Metrics and Evaluation. Global Health Data Echange (GHDx) 2023. Institute for Health Metrics and Evaluation https://vizhub.healthdata.org/gbd-results.
-
- World Health Organization (WHO) Key Facts about Depressive Disorder (depression) 2023. https://www.who.int/fr/news-room/fact-sheets/detail/depression .
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

