Porous metal materials for applications in orthopedic field: A review on mechanisms in bone healing
- PMID: 40226784
- PMCID: PMC11993841
- DOI: 10.1016/j.jot.2024.08.003
Porous metal materials for applications in orthopedic field: A review on mechanisms in bone healing
Abstract
Background: Porous metal materials have been widely studied for applications in orthopedic field, owing to their excellent features and properties in bone healing. Porous metal materials with different compositions, manufacturing methods, and porosities have been developed. Whereas, the systematic mechanisms on how porous metal materials promote bone healing still remain unclear.
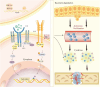
Methods: This review is concerned on the porous metal materials from three aspects with accounts of specific mechanisms, inflammatory regulation, angiogenesis and osteogenesis. We place great emphasis on different cells regulated by porous metal materials, including mesenchymal stem cells (MSCs), macrophages, endothelial cells (ECs), etc.
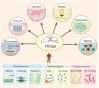
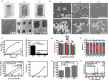
Result: The design of porous metal materials is diversified, with its varying pore sizes, porosity material types, modification methods and coatings help researchers create the most experimentally suitable and clinically effective scaffolds. Related signal pathways presented from different functions showed that porous metal materials could change the behavior of cells and the amount of cytokines, achieving good influence on osteogenesis.
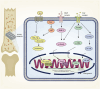
Conclusion: This article summarizes the current progress achieved in the mechanism of porous metal materials promoting bone healing. By modulating the cellular behavior and physiological status of a spectrum of cellular constituents, such as macrophages, osteoblasts, and osteoclasts, porous metal materials are capable of activating different pathways and releasing regulatory factors, thus exerting pivotal influence on improving the bone healing effect.
The translational potential of this article: Porous metal materials play a vital role in the treatment of bone defects. Unfortunately, although an increasing number of studies have been concentrated on the effect of porous metal materials on osteogenesis-related cells, the comprehensive regulation of porous metal materials on the host cell functions during bone regeneration and the related intrinsic mechanisms remain unclear. This review summarizes different design methods for porous metal materials to fabricate the most suitable scaffolds for bone remodeling, and systematically reviews the corresponding mechanisms on inflammation, angiogenesis and osteogenesis of porous metal materials. This review can provide more theoretical framework and innovative optimization for the application of porous metal materials in orthopedics, dentistry, and other areas, thereby advancing their clinical utility and efficacy.
Keywords: Angiogenesis; Bone regeneration; Immunomodulation; Osteogenesis; Porous metal materials.
© 2024 The Authors.
Conflict of interest statement
All authors declare that there are no competing interests.
Figures




References
-
- Chen W., Fang R. [Internal fixation of mandibular comminuted fracture with mini-titanium plate: a retrospective study of 21 cases] Shang Hai Kou Qiang Yi Xue. 2020;29(3):333–336. - PubMed
-
- Won A.M., Montgomery P., Aponte-Wesson R., Chambers M. Implant-supported and magnet-retained oral-nasal combination prosthesis in a patient with a total rhinectomy and partial maxillectomy due to cancer: a clinical report. J Prosthet Dent. 2017;117(2):315–320. - PubMed
-
- Li D., Li Y., Shrestha A., Wang S., Wu Q., Li L., et al. Effects of programmed local delivery from a micro/nano-hierarchical surface on titanium implant on infection clearance and osteogenic induction in an infected bone defect. Adv Healthcare Mater. 2019;8(11) - PubMed
-
- Chang C., Greenspan A., Gershwin M.E. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. 2020;110 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

