What Are the Experiences, Views and Perceptions of Patients, Carers and Clinicians of Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs)? A Scoping Review
- PMID: 40227008
- PMCID: PMC11995417
- DOI: 10.1111/hex.70251
What Are the Experiences, Views and Perceptions of Patients, Carers and Clinicians of Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs)? A Scoping Review
Abstract
Background: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a pharmacological treatment option for both diabetes and weight loss. Qualitative evidence is vital in providing greater understanding of patients, practitioners and carers experience of taking or delivering GLP-1 RAs. This evidence can inform the current or future configuration and delivery of services. We conducted a scoping review to better understand the quantity, nature and key characteristics of qualitative primary evidence which explores the experiences, views and perceptions of patients, carers and clinicians regarding the use of GLP-1 RAs.
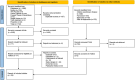
Methods: Four bibliographic databases were searched on 10 July 2024: MEDLINE, APA PsycInfo via Ovid, CINAHL Ultimate via EBSCOhost, ProQuest Dissertations and Theses Global via ProQuest. We also searched Google Scholar, two clinical trials registries, the pre-print server medRxiv and conducted citation searches. We sought qualitative research about the experiences of patients, carers and practitioners about any aspect of taking or prescribing GLP-1RAs, for any indication. Study selection and data extraction were performed by two independent reviewers. The included studies were collated, and their characteristics were described.
Results: After de-duplication 1545 titles and abstracts were screened for relevance, with 77 full-text articles assessed for eligibility, resulting in 25 included studies. More studies were focused on type 2 diabetes (n = 12) than weight loss (n = 9) or any indication (n = 4). The experiences of carers were not represented. No one area of experience (e.g. different indications or viewpoints) was well represented, either due to the absence or narrow focus of studies or lack of an in-depth analytical approach.
Conclusion: Whilst primary qualitative evidence exploring patient and clinician experience of GLP-1 RAs was identified in this scoping review, the findings highlight a need for more robust qualitative research to be conducted across all user groups, in particular involving carers, and especially for the indication of weight loss within service settings. This evidence gap needs to be urgently addressed to ensure GLP-1 RAs are appropriately prescribed and patients and carers receive support from services suited to their needs.
Patient or public contribution: Seventeen public collaborators contributed to the search by suggesting additional search terms, helping define the population for inclusion and contributing to protocol development. Their thoughts on the findings of the review helped form the basis for the discussion of this paper.
Keywords: diabetes; glucagon‐Like peptide 1 receptor agonists (GLP‐1 RAs); obesity; qualitative; scoping review; weight loss.
© 2025 The Author(s). Health Expectations published by John Wiley & Sons Ltd.
Conflict of interest statement
Fifteen of the 25 studies reported on conflict of interest [16, 19, 20, 22, 24, 25, 26, 29, 31, 33, 35, 37, 38, 39, 40] and out of these eleven declared some form of conflict [16, 19, 20, 24, 26, 29, 33, 35, 37, 38, 39]. Details of funding sources were reported in 20 studies [16, 17, 18, 19, 20, 21, 22, 24, 25, 26, 28, 29, 31, 33, 34, 35, 36, 38, 39, 40] and seven of these were partially or fully funded by a pharmaceutical company [16, 18, 21, 24, 33, 35, 38]. Ten studies did not include any conflict‐of‐interest statement [15, 16, 19, 21, 25, 26, 28, 30, 32, 34].
The authors declare no conflicts of interest.
Figures
References
-
- World Health Organization (WHO) , Obesity and Overweight (World Health Organization, 2024), accessed 22 May 2024, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
-
- National Institute for Health and Clinical Excellence (NICE) , Tirzepatide for Treating Type 2 Diabetes. Technology Appraisal Guidance [TA924] (National Institute for Health and Clinical Excellence (NICE), 2023), accessed 2 November 2023, https://www.nice.org.uk/guidance/TA924.
-
- National Institute for Health and Clinical Excellence (NICE) , Semaglutide for Managing Overweight and Obesity. Technology Appraisal Guidance [TA875] (National Institute for Health and Clinical Excellence (NICE), 2023), accessed 22 May 2024, https://www.nice.org.uk/guidance/ta875. - PubMed
-
- National Institute for Health and Clinical Excellence (NICE) , Liraglutide for Managing Overweight and Obesity. Technology Appraisal Guidance [TA664] (London: National Institute for Health and Clinical Excellence (NICE), 2020), accessed 22 May 2024, https://www.nice.org.uk/guidance/ta664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous