Combating yellow fever virus with 7-deaza-7-fluoro-2'-C-methyladenosine
- PMID: 40227063
- PMCID: PMC12057363
- DOI: 10.1128/aac.01889-24
Combating yellow fever virus with 7-deaza-7-fluoro-2'-C-methyladenosine
Abstract
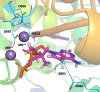
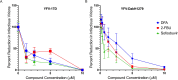
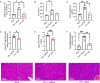
Yellow fever virus (YFV) is a deadly zoonotic flavivirus endemic in tropical/sub-tropical Africa and South America transmitted by mosquito vector (Aedes aegypti; Haemagogus leucocelaenus) to humans and non-human primates. There are no approved antiviral agents for YFV. We previously identified 7-deaza-7-fluoro-2'-C-methyladenosine (DFA) with anti-YFV activity. Interestingly, DFA exhibits pan-activity in vitro against flaviviruses, such as dengue, Japanese encephalitis, Zika, and hepatitis C. This study aimed to expand DFA's anti-flavivirus profile. DFA exhibited potent sub-micromolar anti-YFV activity in vitro against both the vaccine strain (YFV-17D) and a viscerotropic clinical YFV isolate (DakH1279) concomitantly with low cellular cytotoxicity and no notable mitochondrial toxicity. In vivo, efficacy was assessed against both YFV-17DD and a human clinical isolate in A129 and AG129 mouse flavivirus infection models, respectively. DFA significantly reduced virus replication in the livers of YFV-infected mice and the hallmarks of YFV-induced liver damage, including alanine transaminase levels and indocyanine green clearance. Collectively, this work identifies DFA as a potent YFV inhibitor and lays the groundwork for further therapeutic development as a YFV and, potentially, pan-flavivirus therapeutic.
Keywords: 2-FBU; DFA; antiviral agents; flavivirus; nucleoside analogs; yellow fever virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
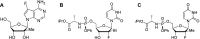

References
-
- Organization PAHO-WH . 2018. Epidemiological update: yellow fever. Available from: https://www3.paho.org/hq/index.php?option=com_docman&view=download&categ...
-
- Organization WH . 2017. World health organization initiative: eliminate yellow fever (eye) epidemics 2017 – 2026. Available from: https://www.who.int/initiatives/eye-strategy#:~:text=The%20EYE%20strateg...
-
- Prevention CfDCa . Yellow fever. https://www.cdc.gov/yellow-fever/index.html.
-
- Gubler DJ, Kuno G, Markoff L. 2007. Flaviviruses, p 1153–1252. In Knipe DM, Howey P (ed), Fields virology. Vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
- P51 OD011092/OD/NIH HHS/United States
- CNPQ: 465425 /2014-3/National Institute of Science and Technology in Dengue and Host-Microorganism Interaction
- 1R21-AI-146503/NH/NIH HHS/United States
- P51 OD011092, R42 AI155275/NH/NIH HHS/United States
- BPD-01010-22,6205283B-BB28-4F9C-AA65-808FE4450542/Fundacao de Amparo a Pesquisa do Estado de Minas Gerais
LinkOut - more resources
Full Text Sources

