Natural Antioxidants Reduce Oxidative Stress and the Toxic Effects of RNA-CUG(exp) in an Inducible Glial Myotonic Dystrophy Type 1 Cell Model
- PMID: 40227219
- PMCID: PMC11939792
- DOI: 10.3390/antiox14030260
Natural Antioxidants Reduce Oxidative Stress and the Toxic Effects of RNA-CUG(exp) in an Inducible Glial Myotonic Dystrophy Type 1 Cell Model
Abstract
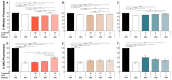
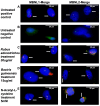
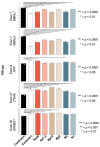
The toxic gain-of-function of RNA-CUG(exp) in DM1 has been largely studied in skeletal muscle, with little focus on its effects on the central nervous system (CNS). This study aimed to study if oxidative stress is present in DM1, its relationship with the toxic RNA gain-of-function and if natural antioxidants can revert some of the RNA-CUG(exp) toxic effects. Using an inducible glial DM1 model (MIO-M1 cells), we compared OS in expanded vs. unexpanded cells and investigated whether antioxidants can mitigate OS and RNA-CUG(exp) toxicity. OS was measured via superoxide anion and lipid peroxidation assays. RNA foci were identified using FISH, and the mis-splicing of selected exons was analyzed using semi-quantitative RT-PCR. Cells were treated with natural antioxidants, and the effects on OS, foci formation, and mis-splicing were compared between treated and untreated cells. The results showed significantly higher superoxide anion and lipid peroxidation levels in untreated DM1 cells, which decreased after antioxidant treatment (ANOVA, p < 0.001). Foci were present in 51% of the untreated cells but were reduced in a dose-dependent manner following treatment (ANOVA, p < 0.001). Antioxidants also improved the splicing of selected exons (ANOVA, p < 0.001), suggesting OS plays a role in DM1, and antioxidants may offer therapeutic potential.
Keywords: RNA toxic gain-of-function; antioxidants; glial cell model; inducible model; myotonic dystrophy; treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Harper P.S. Myotonic Dystrophy. 2nd ed. W B Saunders Company; Philadelphia, PA, USA: 1989.
Grants and funding
LinkOut - more resources
Full Text Sources

