Procyanidin A1 from Peanut Skin Exerts Anti-Aging Effects and Attenuates Senescence via Antioxidative Stress and Autophagy Induction
- PMID: 40227314
- PMCID: PMC11939485
- DOI: 10.3390/antiox14030322
Procyanidin A1 from Peanut Skin Exerts Anti-Aging Effects and Attenuates Senescence via Antioxidative Stress and Autophagy Induction
Abstract
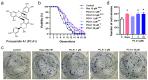
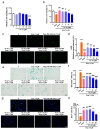
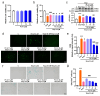
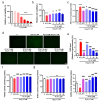
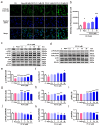
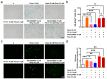
The aging population is steadily increasing, with aging and age-related diseases serving as major risk factors for morbidity, mortality, and economic burden. Peanuts, known as the "longevity nut" in China, have been shown to offer various health benefits, with peanut skin extract (PSE) emerging as a key compound of interest. This study investigates the bioactive compound in PSE with anti-aging potential and explores its underlying mechanisms of action. Procyanidin A1 (PC A1) was isolated from PSE, guided by the K6001 yeast replicative lifespan model. PC A1 prolonged the replicative lifespan of yeast and the yeast-like chronological lifespan of PC12 cells. To further confirm its anti-aging effect, cellular senescence, a hallmark of aging, was assessed. In senescent cells induced by etoposide (Etop), PC A1 alleviated senescence by reducing ROS levels, decreasing the percentage of senescent cells, and restoring proliferative capacity. Transcriptomics analysis revealed that PC A1 induced apoptosis, reduced senescence-associated secretory phenotype (SASP) factors, and modulated the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. The antioxidative capacity of PC A1 was also evaluated, showing enhanced resistance to oxidative stress in PC12 cells by reducing reactive oxygen species (ROS) and malondialdehyde (MDA) levels and increasing superoxide dismutase (SOD) activity. Moreover, PC A1 induced autophagy, as evidenced by an increase in fluorescence-labeled autophagic compartments and confirmation via Western blot analysis of autophagy-related proteins. In addition, the treatment of an autophagy inhibitor abolished the antioxidative stress and senescence-alleviating effects of PC A1. These findings reveal that PC A1 extended lifespans and alleviated cellular senescence by enhancing oxidative stress resistance and inducing autophagy, positioning it as a promising candidate for further exploration as a geroprotective agent.
Keywords: PI3K/Akt signaling pathway; aging; antioxidative stress; autophagy; cell senescence; peanut skin; procyanidin A1.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Isoquercitrin from Apocynum venetum L. Exerts Antiaging Effects on Yeasts via Stress Resistance Improvement and Mitophagy Induction through the Sch9/Rim15/Msn Signaling Pathway.Antioxidants (Basel). 2023 Oct 31;12(11):1939. doi: 10.3390/antiox12111939. Antioxidants (Basel). 2023. PMID: 38001792 Free PMC article.
-
Involvement of the Sch9/Rim15/Msn2 signaling pathway in the anti-aging activity of dendrobine from Dendrobium nobile Lindl. via modification of oxidative stress and autophagy.Chin Med. 2023 Sep 5;18(1):111. doi: 10.1186/s13020-023-00827-4. Chin Med. 2023. PMID: 37670345 Free PMC article.
-
β-Cyclocitral from Lavandula angustifolia Mill. Exerts Anti-Aging Effects on Yeasts and Mammalian Cells via Telomere Protection, Antioxidative Stress, and Autophagy Activation.Antioxidants (Basel). 2024 Jun 12;13(6):715. doi: 10.3390/antiox13060715. Antioxidants (Basel). 2024. PMID: 38929154 Free PMC article.
-
Inokosterone from Gentiana rigescens Franch Extends the Longevity of Yeast and Mammalian Cells via Antioxidative Stress and Mitophagy Induction.Antioxidants (Basel). 2022 Jan 23;11(2):214. doi: 10.3390/antiox11020214. Antioxidants (Basel). 2022. PMID: 35204097 Free PMC article.
-
Arctigenin from Fructus arctii Exhibits Antiaging Effects via Autophagy Induction, Antioxidative Stress, and Increase in Telomerase Activity in Yeast.Antioxidants (Basel). 2024 Jun 2;13(6):684. doi: 10.3390/antiox13060684. Antioxidants (Basel). 2024. PMID: 38929123 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

