diAcCA, a Pro-Drug for Carnosic Acid That Activates the Nrf2 Transcriptional Pathway, Shows Efficacy in the 5xFAD Transgenic Mouse Model of Alzheimer's Disease
- PMID: 40227330
- PMCID: PMC11939361
- DOI: 10.3390/antiox14030293
diAcCA, a Pro-Drug for Carnosic Acid That Activates the Nrf2 Transcriptional Pathway, Shows Efficacy in the 5xFAD Transgenic Mouse Model of Alzheimer's Disease
Abstract
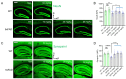
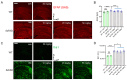
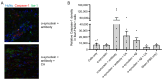
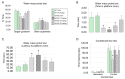
The antioxidant/anti-inflammatory compound carnosic acid (CA) is a phenolic diterpene found in the herbs rosemary and sage. Upon activation, CA manifests electrophilic properties to stimulate the Nrf2 transcriptional pathway via reaction with Keap1. However, purified CA is readily oxidized and thus highly unstable. To develop CA as an Alzheimer's disease (AD) therapeutic, we synthesized pro-drug derivatives, among which the di-acetylated form (diAcCA) showed excellent drug-like properties. diAcCA converted to CA in the stomach prior to absorption into the bloodstream, and exhibited improved stability and bioavailability as well as comparable pharmacokinetics (PK) and efficacy to CA. To test the efficacy of diAcCA in AD transgenic mice, 5xFAD mice (or littermate controls) received the drug for 3 months, followed by behavioral and immunohistochemical studies. Notably, in addition to amyloid plaques and tau tangles, a hallmark of human AD is synapse loss, a major correlate to cognitive decline. The 5xFAD animals receiving diAcCA displayed synaptic rescue on immunohistochemical analysis accompanied by improved learning and memory in the water maze test. Treatment with diAcCA reduced astrocytic and microglial inflammation, amyloid plaque formation, and phospho-tau neuritic aggregates. In toxicity studies, diAcCA was as safe or safer than CA, which is listed by the FDA as "generally regarded as safe", indicating diAcCA is suitable for human clinical trials in AD.
Keywords: Alzheimer’s disease; Nrf2 transcriptional pathway; carnosic acid; therapeutic drug for neurodegeneration.
Conflict of interest statement
Phil S. Baran and Stuart A. Lipton are named inventors on a patent application assigned to their institution, The Scripps Research Institute, covering the chemical entities mentioned in this manuscript. Ravi Natarajan is at Socrates Biosciences, Inc., the company that supplied diAcCA for the work described here. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The other authors declare no conflicts of interest.
Figures


References
Grants and funding
- R56 AG065372/AG/NIA NIH HHS/United States
- R35 AG071734/AG/NIA NIH HHS/United States
- R01 AG078756/AG/NIA NIH HHS/United States
- R01 AG056259/AG/NIA NIH HHS/United States
- S10 OD030332/OD/NIH HHS/United States
- DP1 DA041722/DA/NIDA NIH HHS/United States
- U01 AG088679/AG/NIA NIH HHS/United States
- U01 AG088679, R01 AG056259, R35 AG071734, RF1 AG057409, R01 AG056259, R56 AG065372, R01 DA048882, DP1 DA041722 , S10 OD030332/GF/NIH HHS/United States
- R01 DA048882/DA/NIDA NIH HHS/United States
- RF1 AG057409/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

