The Janus Face of Oxidative Stress and Hydrogen Sulfide: Insights into Neurodegenerative Disease Pathogenesis
- PMID: 40227410
- PMCID: PMC11939184
- DOI: 10.3390/antiox14030360
The Janus Face of Oxidative Stress and Hydrogen Sulfide: Insights into Neurodegenerative Disease Pathogenesis
Abstract
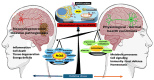
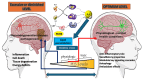
Oxidative stress plays an essential role in neurodegenerative pathophysiology, acting as both a critical signaling mediator and a driver of neuronal damage. Hydrogen sulfide (H2S), a versatile gasotransmitter, exhibits a similarly "Janus-faced" nature, acting as a potent antioxidant and cytoprotective molecule at physiological concentrations, but becoming detrimental when dysregulated. This review explores the dual roles of oxidative stress and H2S in normal cellular physiology and pathophysiology, focusing on neurodegenerative disease progression. We highlight potential therapeutic opportunities for targeting redox and sulfur-based signaling systems in neurodegenerative diseases by elucidating the intricate balance between these opposing forces.
Keywords: Janus face; hydrogen sulfide (H2S); neurodegenerative diseases; neuroinflammation; oxidative stress; redox signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hydrogen Sulfide and Gut Microbiota: Their Synergistic Role in Modulating Sirtuin Activity and Potential Therapeutic Implications for Neurodegenerative Diseases.Pharmaceuticals (Basel). 2024 Nov 4;17(11):1480. doi: 10.3390/ph17111480. Pharmaceuticals (Basel). 2024. PMID: 39598392 Free PMC article. Review.
-
Redox Biology of Hydrogen Sulfide: Implications for Physiology, Pathophysiology, and Pharmacology.Redox Biol. 2013 Jan 1;1(1):32-39. doi: 10.1016/j.redox.2012.11.006. Redox Biol. 2013. PMID: 23795345 Free PMC article.
-
Therapeutic importance of hydrogen sulfide in age-associated neurodegenerative diseases.Neural Regen Res. 2020 Apr;15(4):653-662. doi: 10.4103/1673-5374.266911. Neural Regen Res. 2020. PMID: 31638087 Free PMC article. Review.
-
Role of Microbiota-Derived Hydrogen Sulfide (H2S) in Modulating the Gut-Brain Axis: Implications for Alzheimer's and Parkinson's Disease Pathogenesis.Biomedicines. 2024 Nov 23;12(12):2670. doi: 10.3390/biomedicines12122670. Biomedicines. 2024. PMID: 39767577 Free PMC article. Review.
-
Hydrogen Sulfide and the Kidney: Physiological Roles, Contribution to Pathophysiology, and Therapeutic Potential.Antioxid Redox Signal. 2022 Feb;36(4-6):220-243. doi: 10.1089/ars.2021.0014. Epub 2021 Dec 31. Antioxid Redox Signal. 2022. PMID: 34978847 Review.
Cited by
-
Antioxidants and Reactive Oxygen Species: Shaping Human Health and Disease Outcomes.Int J Mol Sci. 2025 Aug 4;26(15):7520. doi: 10.3390/ijms26157520. Int J Mol Sci. 2025. PMID: 40806653 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

