THEMIS2 contributes to ovarian cancer metastasis via DOCK4-mediated activation of Rap1 signaling
- PMID: 40227532
- PMCID: PMC12238187
- DOI: 10.1007/s13402-025-01057-6
THEMIS2 contributes to ovarian cancer metastasis via DOCK4-mediated activation of Rap1 signaling
Abstract
Purpose: Ovarian cancer (OC) is the most lethal gynecological malignancy, with widespread metastasis and ascites being the leading causes of patient mortality. However, the mechanisms driving OC metastasis have not been sufficiently studied. This study aimed to investigate the mechanisms and key molecules promoting OC metastasis.
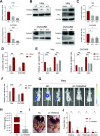
Methods: Public databases (StemChecker, GeneCards, GEO, and TCGA) were screened to identify metastasis-associated genes. Immunohistochemical staining and western blotting were employed to evaluate THEMIS2 expression and epithelial-mesenchymal transition (EMT) marker profiles across experimental groups. RNA sequencing coupled with pathway enrichment analysis revealed THEMIS2-regulated signaling pathways, while immunoprecipitation-mass spectrometry was utilized to identify THEMIS2 interaction partners. GST pull-down assays for active Rap1 quantified Rap1-GTP levels under varying THEMIS2 expression conditions. Wound healing and transwell invasion assays respectively assessed migratory and invasive capacities of OC cells following THEMIS2 expression perturbations in vitro. Abdominal cavity implantation metastasis model was established to evaluate OC cell colonization and invasive potential in vivo.
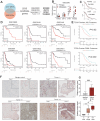
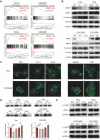
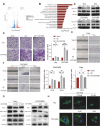
Results: THEMIS2 expression is significantly elevated in OC tissues compared to normal ovarian tissues, and its high expression correlates with poor prognosis and malignant features. Experimental manipulation of THEMIS2 levels revealed that knockdown impended the migratory and invasive capacities of OC cells both in vitro and in vivo, while its overexpression exacerbated metastasis. THEMIS2 is involved in EMT and cytoskeleton rearrangement. RNA-seq analysis revealed that THEMIS2 positively correlates with Rap1 signaling pathway. Inhibition of Rap1 activity reversed the metastasis-promoting effects induced by THEMIS2 overexpression both in vitro and in vivo. Mechanistically, we uncovered that THEMIS2 functions as a molecular scaffold that recruits TBK1 (TANK Binding Kinase 1) to DOCK4 (Dedicator of Cytokinesis 4), facilitating site-specific phosphorylation at serine 1787 (S1787). This post-translational modification enables DOCK4 to engage with CRKII, subsequently triggering Rap1 signaling activation. These findings suggest that THEMIS2 promotes the metastatic potential of OC cells via DOCK4-mediated activation of Rap1 signaling.
Conclusion: THEMIS2 may serve as a predictive biomarker for OC prognosis, and targeting the Rap1 signaling pathway with specific inhibitors represents a promising therapeutic strategy for OC treatment.
Keywords: DOCK4; Ovarian cancer; Rap1 signaling; THEMIS2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in compliance with the Declaration of Helsinki principles. Informed consents were obtained from all the subjects. Ethics approval for human subjects was provided by the Ethics Committee of Fudan University Shanghai Cancer Center. Ethics approval for animal work was provided by the Institutional Animal Care and Use Committee of Shanghai Cancer Center. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- 82002618/National Natural Science Foundation of China
- 81772808/National Natural Science Foundation of China
- 82072876/National Natural Science Foundation of China
- 20YF1407900/Science and Technology Commission of Shanghai Municipality
- 20Y11903500/Science and Technology Commission of Shanghai Municipality
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

