Advanced imaging characterization of post-chemoradiation glioblastoma stratified by diffusion MRI phenotypes known to predict favorable anti-VEGF response
- PMID: 40227555
- PMCID: PMC12170782
- DOI: 10.1007/s11060-025-05019-8
Advanced imaging characterization of post-chemoradiation glioblastoma stratified by diffusion MRI phenotypes known to predict favorable anti-VEGF response
Abstract
Purpose: Recurrent glioblastomas showing a survival benefit from anti-VEGF agents are known to exhibit a distinct diffusion MRI phenotype. We aim to characterize advanced imaging features of this glioblastoma subset.
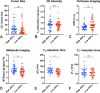
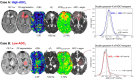
Methods: MRI scans from 87 patients with IDH-wildtype glioblastoma were analyzed. All patients had completed standard chemoradiation and were anti-VEGF-naïve. Contrast-enhancing tumor segmentations were used to extract: the lowest peak of the double gaussian distribution of apparent diffusion coefficient values (ADCL) calculated from diffusion MRI, relative cerebral blood flow (rCBV) values from perfusion MRI, MTRasym @ 3ppm from pH-weighted amine CEST MRI, quantitative T2 and T2* relaxation times (qT2 and qT2*), T1w subtraction map values, and contrast-enhancing tumor volume. Lesions were categorized as high- or low-ADCL using a cutoff of 1240 µm2/s, according to previous studies.
Results: High-ADCL lesions showed significantly lower rCBV (1.02 vs. 1.28, p = 0.0057), higher MTRasym @ 3ppm (2.36% vs. 2.10%, p = 0.0043), and higher qT2 (114.8 ms vs. 100.9 ms, p = 0.0094), compared to low-ADCL lesions. No group differences were seen in contrast-enhancing tumor volume, T1w subtraction map values, and qT2*, nor in clinical variables such as sex category, MGMT status, and EGFR status. Finally, no clear group-specific preferential locations were seen.
Conclusion: Post-chemoradiation glioblastomas with a diffusion MRI phenotype that is known to predict a favorable response to anti-VEGF (ADCL ≥1240 µm2/s) have distinct biological features, with different perfusion and metabolic characteristics, and T2 relaxation times.
Keywords: Anti-VEGF; Anti-angiogenic; Bevacizumab; Diffusion-weighted imaging; Glioblastoma; Perfusion-weighted imaging.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The collection and analysis of clinical and imaging data for this research was approved by the institutional review board under the identification number IRB#19-002084. Competing interests: BME is on the advisory board and is a paid consultant for Medicenna, MedQIA, Servier Pharmaceuticals, Siemens, Janssen Pharmaceuticals, Imaging Endpoints, Kazia, Chimerix, Sumitomo Dainippon Pharma Oncology, ImmunoGenesis, Ellipses Pharma, Monteris, Neosoma, Alpheus Medical, Sagimet Biosciences, Sapience Therapeutics, Orbus Therapeutics, and the Global Coalition for Adap- tive Research (GCAR). TFC is cofounder, major stock holder, consultant and board member of Katmai Pharmaceuticals, holds stock for Erasca, member of the board and paid consultant for the 501c3 Global Coalition for Adaptive Research, holds stock in Chimerix and receives milestone payments and possible future royalties, member of the scientific advisory board for Break Through Cancer, member of the scientific advisory board for Cure Brain Cancer Foundation, has provided paid consulting services to Blue Rock, Vida Ventures, Lista Therapeutics, Stemline, Novartis, Roche, Sonalasense, Sagimet, Clinical Care Options, Ideology Health, Servier, Jubilant, Immvira, Gan & Lee, BrainStorm, Katmai, Sapience, Inovio, Vigeo Therapeutics, DNATrix, Tyme, SDP, Kintara, Bayer, Merck, Boehinger Ingelheim, VBL, Amgen, Kiyatec, Odonate Thera- peutics QED, Medefield, Pascal Biosciences, Bayer, Tocagen, Karyo- pharm, GW Pharma, Abbvie, VBI, Deciphera, VBL, Agios, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Novocure, Novogen, Boston Biomedical, Sunovion, Insys, Pfizer, Notable labs, Medqia, Trizel, Medscape and has contracts with UCLA for the Brain Tumor Program with Roche, VBI, Merck, Novartis, BMS, AstraZeneca, Servier. The Regents of the University of California (T.F.C. employer) has licensed intellectual property co-invented by TFC to Katmai Pharmaceuticals.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

