The Role of Perirenal Adipose Tissue in Carcinogenesis-From Molecular Mechanism to Therapeutic Perspectives
- PMID: 40227577
- PMCID: PMC11987925
- DOI: 10.3390/cancers17071077
The Role of Perirenal Adipose Tissue in Carcinogenesis-From Molecular Mechanism to Therapeutic Perspectives
Abstract
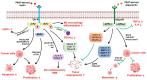
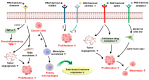
Perirenal adipose tissue (PRAT) exhibits particular morphological features, with its activity being mainly related to thermogenesis. However, an expanded PRAT area seems to play a significant role in cardiovascular diseases, diabetes mellitus, and chronic kidney disease pathogenesis. Numerous studies have demonstrated that PRAT may support cancer progression and invasion, mainly in obese patients. The mechanism underlying these processes is of dysregulation of PRAT's secretion of adipokines and pro-inflammatory cytokines, such as leptin, adiponectin, chemerin, apelin, omentin-1, vistatin, nesfatin-1, and other pro-inflammatory cytokines, modulated by tumor cells. Cancer cells may also induce a metabolic reprogramming of perirenal adipocytes, leading to increased lipids and lactate transfer to the tumor microenvironment, contributing to cancer growth in a hypoxic milieu. In addition, the PRAT browning process has been specifically detected in renal cell carcinoma (RCC), being characterized by upregulated expression of brown/beige adipocytes markers (UCP1, PPAR-ɣ, c/EBPα, and PGC1α) and downregulated white fat cells markers, such as LEPTIN, SHOX2, HOXC8, and HOXC9. Considering its multifaceted role in cancer, modulation of PRAT's role in tumor progression may open new directions for oncologic therapy improvement. Considering the increasing evidence of the relationship between PRAT and tumor cells, our review aims to provide a comprehensive analysis of the perirenal adipocytes' impact on tumor progression and metastasis.
Keywords: adipokines; cancer; cancer therapy; metastasis; perirenal adipose tissue; predictive factor; prognosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Jespersen N.Z., Feizi A., Andersen E.S., Heywood S., Hattel H.B., Daugaard S., Peijs L., Bagi P., Feldt-Rasmussen B., Schultz H., et al. Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol. Metab. 2019;24:30–43. doi: 10.1016/j.molmet.2019.03.005. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

