Guidance for the Clinical Use of the Breast Cancer Polygenic Risk Scores
- PMID: 40227593
- PMCID: PMC11987971
- DOI: 10.3390/cancers17071056
Guidance for the Clinical Use of the Breast Cancer Polygenic Risk Scores
Abstract
Background/Objectives: Polygenic risk scores (PRSs) have been extensively studied and are increasingly applied in healthcare. One of the most studied and developed areas is predictive medicine for breast cancer, but there is no wider consensus on the indications for the clinical use of PRSs for breast cancer. This current guidance endeavours to articulate the scientific evidence underpinning the clinical utility of PRSs in stratifying breast cancer risk, with a particular emphasis on clinical application. Methods: This guidance has been prepared by a group of experts who have been active in breast cancer PRS research and development, combining a review of the evidence base with expert opinion for indications for clinical use. Results: Based on data from various studies and existing breast cancer prevention and screening services, the indications for clinical use of breast cancer PRSs can be divided into the following scenarios: (1) Management of cancer-free women with a family history of cancer; (2) individual personalised breast cancer prevention and screening in healthcare services; and (3) breast cancer screening programs for more personalised screening. Conclusions: The integration of PRSs into clinical practice enables healthcare providers to deliver more accurate risk assessments, personalised prevention strategies, and optimised screening programmes, thereby improving patient outcomes and enhancing the effectiveness of breast cancer care. PRS testing represents a novel component in clinical breast cancer risk assessment, supporting a personalised, risk-based approach to breast cancer prevention and screening.
Keywords: breast cancer; genetic predisposition; personalised medicine; polygenic risk score; prevention; screening.
Conflict of interest statement
Peeter Padrik has ownership in OÜ Antegenes. D Gareth Evans is an advisor to OÜ Antegenes. Siim Sõber owns an option for shares in OÜ Antegenes. Krista Kruuv-Käo owns an option for shares in OÜ Antegenes. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
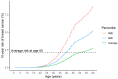
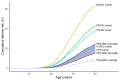
References
-
- Abu-El-Haija A., Reddi H.V., Wand H., Rose N.C., Mori M., Qian E., Murray M.F., Practice A.P., The ACMG Professional Practice and Guidelines Committee The clinical application of polygenic risk scores: A points to consider statement of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. Off. J. Am. Coll. Med. Genet. 2023;25:100803. doi: 10.1016/j.gim.2023.100803. - DOI - PubMed
-
- AnteNOR Project. [(accessed on 1 October 2024)]. Available online: https://antegenes.com/antenor/
-
- BRIGHT Project. [(accessed on 1 October 2024)]. Available online: https://brightscreening.eu.
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer; Lyon, France: 2018. [(accessed on 15 September 2024)]. Available online: https://gco.iarc.fr/today.
-
- NCCN Clinical Practice Guidelines in Oncology Breast Cancer Risk Reduction. Version 2. 2024. [(accessed on 30 May 2024)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf.
Publication types
Grants and funding
- project #230121/BRIGHT consortium by European Commission via EIT Health BRIGHT innovation activity
- Project No 2014-2021.1.02.20-0113/The AnteNOR consortium was sponsoring the development of the guidance through a grant from the Norwegian Grants 2014-2021 Green ICT programme
- IS-BRC-1215-20007/D Gareth Evans is supported by the Manchester National Institute for Health Research (NIHR) Biomedical Research Centre
- PSG774/Sander Pajusalu is supported by the Estonian Research Council grant
LinkOut - more resources
Full Text Sources

