Exploratory Study to Evaluate the Impact of Interim PET/CT Assessment in First-Line Follicular Lymphoma
- PMID: 40227617
- PMCID: PMC11988115
- DOI: 10.3390/cancers17071065
Exploratory Study to Evaluate the Impact of Interim PET/CT Assessment in First-Line Follicular Lymphoma
Abstract
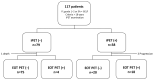
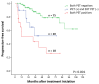
Background/Objectives: This study aimed to determine whether interim PET/CT (iPET) scans could identify follicular lymphoma (FL) patients at high risk of relapse following first-line therapy. Methods: A total of 117 FL patients who underwent iPET scans were included, with responses interpreted using the Deauville score (DS). Progression-free survival (PFS) was evaluated over a median follow-up of 34 months. Results: Overall, 34% of patients were classified as iPET (+), with significantly worse estimated 5-year PFS compared to iPET (-) patients (34% vs. 76%, hazard ratio 4.3, p < 0.001). Multivariate analysis confirmed iPET (+) as an independent predictor of PFS. Conclusions: Interim PET results are significant predictors of PFS in FL first-line therapy and could inform response-adapted treatment strategies.
Keywords: PET/CT; follicular lymphoma 2; interim evaluation.
Conflict of interest statement
Alejandro Martin-Muñoz, is an employee of Altum Sequencing Co. Rosa Ayala and Joaquín Martínez are equity shareholders of Altum Sequencing Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


References
-
- Cheah C.Y., Chihara D., Horowitz S., Sevin A., Oki Y., Zhou S., Fowler N.H., Romaguera J.E., Turturro F., Hagemeister F.B., et al. Factors influencing outcome in advanced stage, low-grade follicular lymphoma treated at MD Anderson Cancer Center in the rituximab era. Ann. Oncol. 2016;27:895–901. - PubMed
-
- Jiménez-Ubieto A., Grande C., Caballero D., Yáñez L., Novelli S., Hernández-Garcia M.T., Manzanares M., Arranz R., Ferreiro J.J., Bobillo S., et al. Autologous stem cell transplantation for follicular lymphoma: Favorable long-term survival irrespective of pretransplantation rituximab exposure. Biol. Blood Marrow Transplant. 2017;23:1631–1640. - PubMed
-
- Anderson J.R., Armitage J.O., Weisenburger D.D. Epidemiology of the non-Hodgkin’s lymphomas: Distributions of the major subtypes differ by geographic locations. Ann. Oncol. 1998;9:717–720. - PubMed
-
- Bastos-Oreiro M., Muntañola A., Panizo C., Gonzalez-Barca E., de Villambrosia S.G., Córdoba R., López J.L.B., González-Sierra P., Terol M.J., Gutierrez A., et al. RELINF: Prospective epidemiological registry of lymphoid neoplasms in Spain. A project from the GELTAMO group. Ann. Hematol. 2020;99:799–808. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

