The Application of Liquid Biopsy for the Development and Validation of a Non-Invasive Screening and Diagnosis Test for Endometrial Premalignant and Malignant Lesions: A Prospective Innovative Pilot Study
- PMID: 40227624
- PMCID: PMC11988008
- DOI: 10.3390/cancers17071078
The Application of Liquid Biopsy for the Development and Validation of a Non-Invasive Screening and Diagnosis Test for Endometrial Premalignant and Malignant Lesions: A Prospective Innovative Pilot Study
Abstract
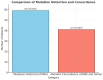
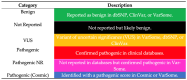
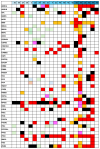
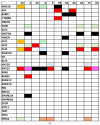
Background/Objectives: Endometrial cancer (EC) is a common malignancy in developed countries, with incidence closely linked to lifestyle factors and genetic predispositions, notably Lynch syndrome. Traditional biopsy methods for diagnosis and monitoring are invasive. This study aims to develop and validate a non-invasive diagnostic method for EC using liquid biopsy, specifically examining circulating tumor DNA (ctDNA) for its potential in early detection and disease monitoring. Methods: A cohort of 63 patients with EC or atypical endometrial hyperplasia (AEH) was recruited from the Gynecological Unit of the Azienda Ospedaliera Universitaria Federico II. Plasma samples were processed to extract ctDNA, which was sequenced and analyzed for mutations. Matched tumor tissue and germline DNA were also examined to confirm mutation concordance and assess potential genetic predispositions. Results: Pathogenic mutations were identified in plasma ctDNA in 59 out of 63 cases (93%), with a 65% concordance between plasma ctDNA mutations and those found in solid tumor samples. Key mutations in genes such as PTEN, PIK3R1, and KMT2C were significantly associated with a higher tumor grade and advanced stage disease, such as myometrial infiltration. Conclusions: Liquid biopsy shows promise as a minimally invasive diagnostic and monitoring tool for EC, offering real-time insights into tumor biology. The high mutation concordance between the plasma ctDNA and tumor tissue underscores the potential of a liquid biopsy in managing EC, particularly for patients at risk of recurrence. Further longitudinal studies are needed to establish ctDNA as a standard tool in EC diagnosis and monitoring.
Keywords: cancer monitoring; circulating tumor DNA; endometrial cancer; liquid biopsy; next-generation sequencing; non-invasive diagnostics; personalized medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
High concordance of actionable genomic alterations identified between circulating tumor DNA-based and tissue-based next-generation sequencing testing in advanced non-small cell lung cancer: The Korean Lung Liquid Versus Invasive Biopsy Program.Cancer. 2021 Aug 15;127(16):3019-3028. doi: 10.1002/cncr.33571. Epub 2021 Apr 7. Cancer. 2021. PMID: 33826761
-
Circulating tumor DNA as a prognostic marker in high-risk endometrial cancer.J Transl Med. 2021 Feb 3;19(1):51. doi: 10.1186/s12967-021-02722-8. J Transl Med. 2021. PMID: 33536036 Free PMC article.
-
Concordance of Circulating Tumor DNA and Matched Metastatic Tissue Biopsy in Prostate Cancer.J Natl Cancer Inst. 2017 Dec 1;109(12):djx118. doi: 10.1093/jnci/djx118. J Natl Cancer Inst. 2017. PMID: 29206995 Free PMC article.
-
Circulating tumor DNA in endometrial cancer: clinical significance and implications.Int J Gynecol Cancer. 2025 Apr;35(4):101656. doi: 10.1016/j.ijgc.2025.101656. Epub 2025 Jan 23. Int J Gynecol Cancer. 2025. PMID: 39955181 Review.
-
Liquid Biopsy for Monitoring EC Patients: Towards Personalized Treatment.Cancers (Basel). 2022 Mar 9;14(6):1405. doi: 10.3390/cancers14061405. Cancers (Basel). 2022. PMID: 35326558 Free PMC article. Review.
Cited by
-
Beyond the Microscope: Integrating Liquid Biopsies into the Molecular Pathology Era of Endometrial Cancer.Int J Mol Sci. 2025 Aug 19;26(16):7987. doi: 10.3390/ijms26167987. Int J Mol Sci. 2025. PMID: 40869308 Free PMC article. Review.
References
-
- Colombo N., Creutzberg C., Amant F., Bosse T., González-Martín A., Ledermann J., Marth C., Nout R., Querleu D., Mirza M.R., et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int. J. Gynecol. Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. - DOI - PMC - PubMed
-
- ECIS—European Cancer Information System Homepage. [(accessed on 14 November 2024)]. Available online: https://ecis.jrc.ec.europa.eu/en.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

