Uterine Pgrmc2 Deficiency Attenuates Endometrial Hyperplasia and Cancer and Prolongs Lifespan in a Pten Loss-of-Function-Induced Cancer Model
- PMID: 40227710
- PMCID: PMC11988108
- DOI: 10.3390/cancers17071178
Uterine Pgrmc2 Deficiency Attenuates Endometrial Hyperplasia and Cancer and Prolongs Lifespan in a Pten Loss-of-Function-Induced Cancer Model
Abstract
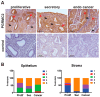
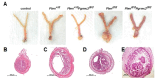
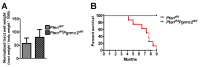
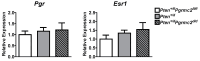
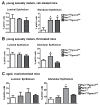
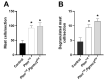
The expression of members of the progesterone receptor membrane component (PGRMC) family, particularly PGRMC1, is elevated in diverse types of cancers, particularly those of the female reproductive system. While xenograft tumor studies using human transformed cell lines in immunocompromised mice have suggested that PGRMC1 enhances tumor growth and chemoresistance, the exact role of members of the PGRMC family in cancer development in vivo remains unclear. In this study, we examined the effect of deleting Pgrmc2 on the development of endometrial hyperplasia and cancer using a murine phosphatase and tensin homologue (Pten) conditional loss-of-function model. We previously established that PGRMCs are cell survival factors that are required for normal estrogen-induced uterine epithelial cell proliferation and normal female fertility. The deletion of Pgrmc2 reduced the incidence and severity of endometrial hyperplasia and cancer in mice with conditional Pten-heterozygous uteri and increased lifespan in mice with conditional Pten-knockout uteri. Mechanistically, the deletion of Pgrmc2 decreased uterine glandular epithelial cell proliferation. Pten loss-of-function-induced endometrial hyperplasia and cancer elevated uterine inflammation, but this was not impacted by PGRMC2 deficiency. This study identifies PGRMC2 as a potential therapeutic target to be inhibited in the treatment of endometrial hyperplasia and cancer, particularly where PTEN activity is reduced due to gene mutation or loss.
Keywords: PGRMC1; PGRMC2; PTEN; cancer; endometrial; hyperplasia; progesterone; uterus.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

References
-
- [(accessed on 1 January 2025)]. Available online: https://www.cancer.org/cancer/types/endometrial-cancer/about/key-statist....
-
- Wallace A.E., Gibson D.A., Saunders P.T.K., Jabbour H.N. Inflammatory events in endometrial adenocarcinoma. J. Endocrinol. 2010;206:141–157. - PubMed
-
- Kong D., Suzuki A., Zou T.T., Sakurada A., Kemp L.W., Wakatsuki S., Yokoyama T., Yamakawa H., Furukawa T., Sato M., et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat. Genet. 1997;17:143–144. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

