Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events
- PMID: 40227712
- PMCID: PMC11988032
- DOI: 10.3390/cancers17071147
Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events
Abstract
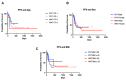
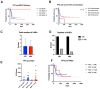
Changes in the dosing schedules for immune checkpoint inhibitors, specifically nivolumab and pembrolizumab, in the treatment of metastatic melanoma, were introduced based on pharmacokinetic data and analysis of pre-existing clinical trial data in the absence of new clinical trials. Therefore, we sought to provide real-world data examining whether fixed-dose therapy (FDT) or weight-adapted therapy (WAT) influenced progression-free (PFS) and overall survival (OS), and the incidence of immune-related adverse events (irAEs). The electronic case notes of all patients (n = 77) treated with immune checkpoint inhibitor immunotherapy (ICI) in the first-line setting for melanoma in the Department of Dermatology, University of Luebeck, between the 1 January 2017 and the 31 December 2020, were retrospectively analysed. Although a higher proportion of patients in the WAT cohort were treated in the palliative setting, there were no correlations between dosing schedule, renal function, or BMI and PFS. Moreover, there were no differences between the cohorts in terms of PFS, OS, or the number and nature of irAEs. An elevated serum S100 concentration was associated with a decreased mean PFS in the FDT cohort (p < 0.001). This study, although inherently limited by its retrospective and monocentric nature, provides reassuring evidence that dosing schedule and pre-existing comorbidities do not influence efficacy or the irAE profile of ICI therapy in the management of melanoma.
Keywords: Real World Data; cancer; fixed dose; immunotherapy; melanoma.
Conflict of interest statement
EAL has received speaker’s honoraria, travel support, and contributed to advisory boards for Novartis, BMS and Sun Pharma. The authors declare no other conflicts of interest.
Figures
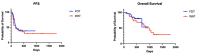
References
-
- Willsmore Z.N., Coumbe B.G.T., Crescioli S., Reci S., Gupta A., Harris R.J., Chenoweth A., Chauhan J., Bax H.J., McCraw A., et al. Combined Anti-Pd-1 and Anti-Ctla-4 Checkpoint Blockade: Treatment of Melanoma and Immune Mechanisms of Action. Eur. J. Immunol. 2021;51:544–556. doi: 10.1002/eji.202048747. - DOI - PubMed
-
- van Zeijl M.C.T., van Breeschoten J., de Wreede L.C., Wouters M., Hilarius D.L., Blank C.U., Aarts M.J.B., van den Berkmortel F., de Groot J.W.B., Hospers G.A.P., et al. Real-world Outcomes of Ipilimumab Plus Nivolumab Combination Therapy in a Nation-Wide Cohort of Advanced Melanoma Patients in the Netherlands. J. Immunother. 2023;46:197–204. doi: 10.1097/CJI.0000000000000468. - DOI - PubMed
LinkOut - more resources
Full Text Sources

