Exosome-Mediated Cellular Communication in the Tumor Microenvironment Imparts Drug Resistance in Breast Cancer
- PMID: 40227747
- PMCID: PMC11987792
- DOI: 10.3390/cancers17071167
Exosome-Mediated Cellular Communication in the Tumor Microenvironment Imparts Drug Resistance in Breast Cancer
Abstract
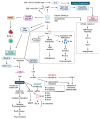
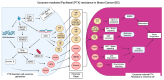
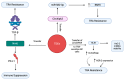
Globally, breast cancer (BC) is the leading cause of cancer-related death for women. BC is characterized by heterogeneity, aggressive behavior, and high metastatic potential. Chemotherapy, administered as monotherapy or adjuvant therapy, remains a cornerstone of treatment; however, acquired drug resistance is a significant clinical challenge. Deciphering mechanisms of drug resistance will be central to developing more efficient treatment options and improving patient outcomes. The current review examines the multifaceted nature of exosomes in conferring drug resistance in BC through complex communication networks within the tumor microenvironment. We further explore recent advances in understanding how exosomes contribute to resistance against established chemotherapeutic agents such as tamoxifen, paclitaxel, doxorubicin, platinum-based drugs, trastuzumab, and newer immunotherapies, such as immune checkpoint inhibitors. Moreover, we discuss existing systematic approaches to investigating the exosome-drug resistance relationship in BC. Finally, we explore promising therapeutic approaches to overcome exosome-dependent drug resistance in BC, highlighting potential avenues for improved treatment efficacy. Investigating the distinct functions and cargo of exosomes offers potential for developing innovative approaches to overcoming treatment resistance.
Keywords: breast cancer; drug resistance; drug sensitization; exosomes; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ugai T., Sasamoto N., Lee H.Y., Ando M., Song M., Tamimi R.M., Kawachi I., Campbell P.T., Giovannucci E.L., Weiderpass E., et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022;19:656–673. doi: 10.1038/s41571-022-00672-8. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

