Pro-Inflammatory Cytokines Transactivate Glycosylated Cytokine Receptors on Cancer Cells to Induce Epithelial-Mesenchymal Transition to the Metastatic Phenotype
- PMID: 40227834
- PMCID: PMC11988151
- DOI: 10.3390/cancers17071234
Pro-Inflammatory Cytokines Transactivate Glycosylated Cytokine Receptors on Cancer Cells to Induce Epithelial-Mesenchymal Transition to the Metastatic Phenotype
Abstract
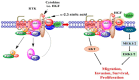
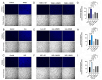
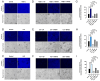
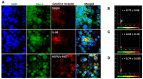
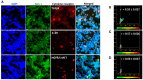
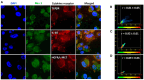
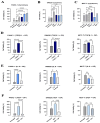
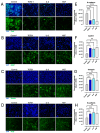
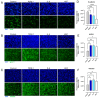
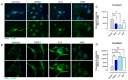
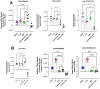
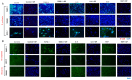
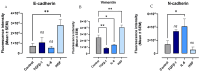
Background/Objectives: The significance of cytokine signaling on cancer progression and metastasis has raised interest in cancer research over the last few decades. Here, we analyzed the effects of three cytokines that we previously reported are significantly upregulated rapidly after the surgical removal of primary breast, colorectal, and prostate cancer. We also investigated the regulation of their cognate receptors. Methods: All experiments were conducted using the PANC-1, SW620, and MCF-7 cell lines, treated with three different cytokines (TGF-β1, HGF, and IL-6). The effect of these cytokines on the expression of epithelial-mesenchymal transition (EMT) cell surface markers and neuraminidase-1 activity was measured via fluorescent microscopy and image analysis software. Results: The findings show that these cytokines increase the expression of mesenchymal markers while reducing epithelial markers, corresponding to the EMT process. A strong link between cytokine receptor signaling and the Neu-1-MMP-9-GPCR crosstalk was identified, suggesting that cytokine receptor binding leads to increased Neu-1 activity and subsequent signaling pathway activation. Oseltamivir phosphate (OP) prevented sialic acid hydrolysis by neuraminidase-1 (Neu-1), leading to the downregulation of these signaling cascades. Conclusions: In concert with the previous work revealing the role of Neu-1 in regulating other glycosylated receptors implicated in cancer cell proliferation and EMT, targeting Neu-1 may provide effective treatment against a variety of malignancies. Most significantly, the treatment of patients with specific inhibitors of Neu-1 soon after primary cancer surgery may improve our ability to cure early-stage cancer by inhibiting the EMT process and disrupting the ability of any residual cancer cell population to metastasize.
Keywords: EMT; glycosylated cytokine receptors; neuraminidase-1; oseltamivir phosphate; pro-inflammatory cytokines.
Conflict of interest statement
William W. Harless owns shares in ENCYT and has a commercial interest and/or patents in the work under consideration.
Figures

Similar articles
-
Artificial and Natural Sweeteners Biased T1R2/T1R3 Taste Receptors Transactivate Glycosylated Receptors on Cancer Cells to Induce Epithelial-Mesenchymal Transition of Metastatic Phenotype.Nutrients. 2024 Jun 12;16(12):1840. doi: 10.3390/nu16121840. Nutrients. 2024. PMID: 38931195 Free PMC article.
-
Functional Selectivity of Cannabinoid Type 1 G Protein-Coupled Receptor Agonists in Transactivating Glycosylated Receptors on Cancer Cells to Induce Epithelial-Mesenchymal Transition Metastatic Phenotype.Cells. 2024 Mar 8;13(6):480. doi: 10.3390/cells13060480. Cells. 2024. PMID: 38534324 Free PMC article.
-
Metformin reverses mesenchymal phenotype of primary breast cancer cells through STAT3/NF-κB pathways.BMC Cancer. 2019 Jul 23;19(1):728. doi: 10.1186/s12885-019-5945-1. BMC Cancer. 2019. PMID: 31337349 Free PMC article.
-
Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process.Mol Cell Endocrinol. 2017 Dec 5;457:103-113. doi: 10.1016/j.mce.2016.12.026. Epub 2016 Dec 29. Mol Cell Endocrinol. 2017. PMID: 28042023 Review.
-
Epigenetic Regulation of Inflammatory Cytokine-Induced Epithelial-To-Mesenchymal Cell Transition and Cancer Stem Cell Generation.Cells. 2019 Sep 25;8(10):1143. doi: 10.3390/cells8101143. Cells. 2019. PMID: 31557902 Free PMC article. Review.
References
-
- Owen J.A., Punt J., Stranford S.A., Jones P.P., Kuby J. Kuby Immunology. 7th ed. W.H. Freeman; New York, NY, USA: 2013.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

