Innovative qPCR Algorithm Using Platelet-Derived RNA for High-Specificity and Cost-Effective Ovarian Cancer Detection
- PMID: 40227838
- PMCID: PMC11988175
- DOI: 10.3390/cancers17071251
Innovative qPCR Algorithm Using Platelet-Derived RNA for High-Specificity and Cost-Effective Ovarian Cancer Detection
Abstract
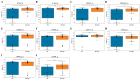
Background/Objectives: Ovarian cancer (OC) remains one of the most lethal gynecologic malignancies, largely due to the challenges of early detection. While next-generation sequencing (NGS) has been explored for screening, its high cost limits large-scale implementation. To develop a more accessible diagnostic solution, we designed a qPCR-based algorithm optimized for early OC detection, with a focus on high-grade serous ovarian cancer (HGSOC), the most aggressive subtype. Methods: Peripheral blood samples from 19 ovarian cancer patients, 37 benign tumor patients, and 34 asymptomatic controls were analyzed using RNA sequencing to identify splice junction-based biomarkers with minimal expression in benign samples but elevated in OC. Results: A final panel of 10 markers was validated via qPCR, demonstrating strong agreement with sequencing data (R2 = 0.44-0.98). The classification algorithm achieved 94.1% sensitivity and 94.4% specificity (AUC = 0.933). Conclusions: By leveraging platelet RNA profiling, this approach offers high specificity, accessibility, and potential for early OC detection. Future studies will focus on expanding histologic diversity and refining biomarker panels to further enhance diagnostic performance.
Keywords: early detection; inflammation; ovarian cancer; platelet RNA; qPCR algorithm.
Conflict of interest statement
Eunyong Ahn, Se Ik Kim, Sungmin Park, Sarah Kim, TaeJin Ahn, and Yong-Sang Song are stockholders of Foretell My Health, Inc. Eunyong Ahn, Sungmin Park, Sarah Kim, Hyunjung Kim, Hyejin Lee, Eun Ji Song, TaeJin Ahn are affiliated with the company Foretell My Health, Inc. We affirm that this conflict of interest does not affect the integrity, objectivity, or validity of the research conducted. All efforts have been made to ensure that the research is conducted impartially and accurately. Patent applications related to some parts of these findings in this study have been filed by the authors.
Figures





References
-
- Menon U., Gentry-Maharaj A., Burnell M., Singh N., Ryan A., Karpinskyj C., Carlino G., Taylor J., Massingham S.K., Raikou M., et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet. 2021;397:2182–2193. doi: 10.1016/S0140-6736(21)00731-5. - DOI - PMC - PubMed
-
- Jacobs I.J., Menon U., Ryan A., Gentry-Maharaj A., Burnell M., Kalsi J.K., Amso N.N., Apostolidou S., Benjamin E., Cruickshank D., et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet. 2016;387:945–956. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

