Dimethyl Itaconate Alleviates Escherichia coli-Induced Endometritis Through the Guanosine-CXCL14 Axis via Increasing the Abundance of norank_f_Muribaculaceae
- PMID: 40227949
- PMCID: PMC12140315
- DOI: 10.1002/advs.202414792
Dimethyl Itaconate Alleviates Escherichia coli-Induced Endometritis Through the Guanosine-CXCL14 Axis via Increasing the Abundance of norank_f_Muribaculaceae
Abstract
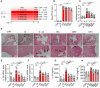
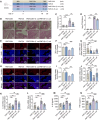
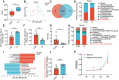
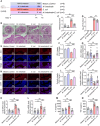
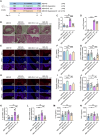
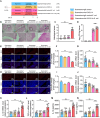
Endometritis, a prevalent reproductive system disease with high incidence, leads to reproductive dysfunction in humans and animals, causing huge economic losses. Dimethyl itaconate (DI) has been demonstrated to exert protective effects in multiple inflammatory diseases. Nevertheless, the efficacy of DI in preventing endometritis and the role played by the gut microbiota remain unknown. In this study, it is found that DI ameliorated Escherichia coli (E. coli) induced endometritis in mice. The protective effect is abolished by antibiotic-induced depletion of the gut microbiota, and fecal microbiota transplantation (FMT) from DI-treated mice to recipient mice ameliorated E. coli-induced endometritis. Integrative multiomics reveals that DI promotes the multiplication of norank_f_Muribaculaceae in vivo, and supplementation of Muribaculum intestinale (DSM 28989), which belongs to the norank_f_Muribaculaceae genus, upregulates the level of guanosine in the uterus. Mechanistically, the protective effect of guanosine in endometritis is mediated by activating the expression of CXCL14 in uterine epithelial cells. Moreover, the antibody-neutralizing experiment of CXCL14 eliminated this protective effect. In conclusion, this study elucidates the significant role of the gut microbiota and its metabolites in the protection of DI against endometritis, and provides new evidence for the regulation of distal organ by the gut microbiota.
Keywords: dimethyl itaconate; endometritis; gut microbiota; gut‐uterus axis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dietary Tryptophan-Mediated Aryl Hydrocarbon Receptor Activation by the Gut Microbiota Alleviates Escherichia coli-Induced Endometritis in Mice.Microbiol Spectr. 2022 Aug 31;10(4):e0081122. doi: 10.1128/spectrum.00811-22. Epub 2022 Jun 21. Microbiol Spectr. 2022. PMID: 35727038 Free PMC article.
-
Intestinal inflammation exacerbates endometritis through succinate production by gut microbiota and SUCNR1-mediated proinflammatory response.Int Immunopharmacol. 2025 Jan 27;146:113919. doi: 10.1016/j.intimp.2024.113919. Epub 2024 Dec 29. Int Immunopharmacol. 2025. PMID: 39736240
-
Uterine Tissue Metabonomics Combined with 16S rRNA Gene Sequencing To Analyze the Changes of Gut Microbiota in Mice with Endometritis and the Intervention Effect of Tau Interferon.Microbiol Spectr. 2023 Jun 15;11(3):e0040923. doi: 10.1128/spectrum.00409-23. Epub 2023 Apr 17. Microbiol Spectr. 2023. PMID: 37067455 Free PMC article.
-
Gut microbiota mediate the protective effects on endometritis induced by Staphylococcus aureus in mice.Food Funct. 2020 Apr 30;11(4):3695-3705. doi: 10.1039/c9fo02963j. Food Funct. 2020. PMID: 32307472
-
Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows.J Dairy Sci. 2019 Dec;102(12):11786-11797. doi: 10.3168/jds.2019-17106. Epub 2019 Oct 3. J Dairy Sci. 2019. PMID: 31587913 Review.
References
-
- Umar T., Yin B., Umer S., Ma X., Jiang K., Umar Z., Akhtar M., Shaukat A., Deng G., Inflammation 2021, 44, 1683. - PubMed
-
- Ravel J., Moreno I., Simón C., Am. J. Obstet. Gynecol. 2021, 224, 251. - PubMed
-
- Sheldon I. M., Noakes D. E., Rycroft A. N., Pfeiffer D. U., Dobson H., Reproduction 2002, 123, 837. - PubMed
-
- Dohmen M. J., Joop K., Sturk A., Bols P. E., Lohuis J. A., Theriogenology 2000, 54, 1019. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
