The multifaceted roles of the transcriptional coactivator TAZ in extravillous trophoblast development of the human placenta
- PMID: 40228123
- PMCID: PMC12037006
- DOI: 10.1073/pnas.2426385122
The multifaceted roles of the transcriptional coactivator TAZ in extravillous trophoblast development of the human placenta
Abstract
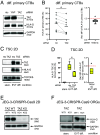
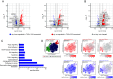
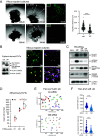
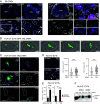
Insights into the molecular processes that drive early development of the human placenta is crucial for our understanding of pregnancy complications such as preeclampsia and fetal growth restriction, since defects in maturation of its epithelial cell, the trophoblast, have been detected in the severe forms of these diseases. However, key regulators specifying the differentiated trophoblast subtypes of the placenta are only slowly emerging. By using diverse trophoblast cell models, we herein show that the transcriptional coactivator of HIPPO signaling, TAZ, plays a pivotal role in the development of invasive extravillous trophoblasts (EVTs), cells that are essential for decidual vessel remodeling and adaption of maternal blood flow to the placenta. Ribonucleic acid sequencing (RNA-seq) or protein analyses upon TAZ gene silencing or CRISPR-Cas9-mediated knockout in differentiating trophoblast stem cells, organoids, primary EVTs, choriocarcinoma cells, or villous explant cultures unraveled that the coactivator promoted expression of genes associated with EVT identity, motility, and survival. Accordingly, depletion or chemical inhibition of TAZ, interacting with TEA domain family member 1 (TEAD1), impaired EVT differentiation, invasion, and migration and triggered apoptosis in the different trophoblast models. Notably, the coactivator also suppressed cell cycle genes and regulators of trophoblast self-renewal and prevented EVTs from cell fusion in organoids and primary cultures. Moreover, TAZ promoted human leukocyte antigen G (HLA-G) surface expression and increased NUAK1 kinase in EVTs thereby maintaining its own expression. In summary, the transcriptional coactivator TAZ plays a multifaceted role in the development of the EVT cell lineage by controlling different biological processes that initiate and preserve differentiation.
Keywords: TAZ; differentiation; extravillous trophoblast; human placenta; trophoblast organoid.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


Similar articles
-
Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta.Proc Natl Acad Sci U S A. 2020 Jun 16;117(24):13562-13570. doi: 10.1073/pnas.2002630117. Epub 2020 Jun 1. Proc Natl Acad Sci U S A. 2020. PMID: 32482863 Free PMC article.
-
Transforming growth factor-β signaling governs the differentiation program of extravillous trophoblasts in the developing human placenta.Proc Natl Acad Sci U S A. 2022 Jul 12;119(28):e2120667119. doi: 10.1073/pnas.2120667119. Epub 2022 Jul 6. Proc Natl Acad Sci U S A. 2022. PMID: 35867736 Free PMC article.
-
Inhibition of YAP/TAZ-TEAD activity induces cytotrophoblast differentiation into syncytiotrophoblast in human trophoblast.Mol Hum Reprod. 2022 Sep 29;28(10):gaac032. doi: 10.1093/molehr/gaac032. Mol Hum Reprod. 2022. PMID: 35993908
-
WNT and NOTCH signaling in human trophoblast development and differentiation.Cell Mol Life Sci. 2022 May 13;79(6):292. doi: 10.1007/s00018-022-04285-3. Cell Mol Life Sci. 2022. PMID: 35562545 Free PMC article. Review.
-
TGFβ signalling: a nexus between inflammation, placental health and preeclampsia throughout pregnancy.Hum Reprod Update. 2024 Jul 1;30(4):442-471. doi: 10.1093/humupd/dmae007. Hum Reprod Update. 2024. PMID: 38519450 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

