Human papillomavirus vaccination at age 9 or 10 years to increase coverage - a narrative review of the literature, United States 2014-2024
- PMID: 40228197
- PMCID: PMC12005419
- DOI: 10.1080/21645515.2025.2480870
Human papillomavirus vaccination at age 9 or 10 years to increase coverage - a narrative review of the literature, United States 2014-2024
Abstract
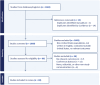
The Advisory Committee on Immunization Practices recommends routine human papillomavirus (HPV) vaccination at 11-12 years; the series can begin at age 9. U.S. HPV vaccination coverage is lower than other adolescent vaccinations. One proposed strategy to increase coverage is initiation at 9-10 years. We systematically reviewed studies addressing vaccination at age 9 to identify and evaluate evidence regarding potential programmatic advantages. Among 30 publications from 2014 to 2024 there were retrospective cohort studies (N = 11), intervention studies with a component focused on vaccination at 9-10 (N = 12), and studies of feasibility or acceptability by providers or caregivers (N = 7). While retrospective analyses found earlier initiation associated with completion, limitations in methodology preclude a cause-and-effect interpretation. Impact of age 9 vaccination is difficult to isolate in intervention studies that had multiple components. While initiating vaccination at age 9 is feasible, questions remain regarding the benefit of this approach to increase coverage.
Keywords: Human papillomavirus; immunization schedule; policy; systematic review; vaccination; vaccination at age 9; vaccine completion; vaccine initiation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56 (RR-2):1–15. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm. - PubMed
-
- Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54(RR–7):1–21. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5407a1.htm. - PubMed
-
- Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Murphy TV, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR03):1–34. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5503a1.htm. - PubMed
-
- Immunization of adolescents . Recommendations of the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, the American Academy of Family Physicians, and the American Medical Association. MMWR Recomm Rep. 1996;45(RR–13):1–16. https://www.cdc.gov/mmwr/preview/mmwrhtml/00044572.htm. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical