Immunotherapy-associated autoimmune hemolytic anemia induced by anti-PD-1 therapy in esophageal cancer: A case report and literature review
- PMID: 40228248
- PMCID: PMC11999398
- DOI: 10.1097/MD.0000000000042174
Immunotherapy-associated autoimmune hemolytic anemia induced by anti-PD-1 therapy in esophageal cancer: A case report and literature review
Abstract
Rationale: Numerous immune checkpoint inhibitors have been approved for clinical use in metastatic advanced esophageal cancer. While immunotherapy brings therapeutic benefits, immune-related adverse events (irAEs) should nevertheless not be overlooked. This paper reports on the first documented case of Autoimmune hemolytic anemia (AIHA) caused by anti-programmed cell death protein-1 therapy in esophageal squamous cancer.
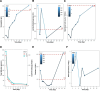
Patient concerns: An 84-year-old female patient with metastatic squamous esophageal cancer developed chest tightness, generalized weakness, and a yellowing of the skin after 2 cycles of sintilimab treatment.
Diagnoses: Initial examination revealed severe anemia with elevated levels of bilirubin, reticulocytes, lactate dehydrogenase, decreased levels of haptoglobin, and a positive direct antihuman globulin test. The patient was diagnosed with immunotherapy-associated AIHA.
Interventions: The patient was promptly treated with corticosteroids and human immunoglobulin, supportive transfusion with washed erythrocytes.
Outcomes: Her AIHA was controlled after treatment. Subsequent immunotherapy was not continued, and there was no recurrence of AIHA.
Lessons: We have identified a rare case of serious adverse reaction caused by anti-PD-1 therapy. We summarize the clinical presentations, diagnosis, and treatment of this case of immunotherapy-related AIHA and discuss the pathogenesis and therapeutic advances in immunotherapy-related AIHA, as well as sintilimab-induced irAEs, in detail. These findings underscore the importance of the early detection, diagnosis, and treatment of these rare and potentially fatal irAEs.
Keywords: autoimmune hemolytic anemia; case report; esophageal cancer; immunotherapy; sintilimab.
Copyright © 2025 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Severe autoimmune hemolytic anemia following immunotherapy with checkpoint inhibitors in two patients with metastatic melanoma: a case report.Front Immunol. 2024 Mar 20;15:1342845. doi: 10.3389/fimmu.2024.1342845. eCollection 2024. Front Immunol. 2024. PMID: 38571955 Free PMC article.
-
Immunotherapy-associated autoimmune hemolytic anemia.J Immunother Cancer. 2017 Feb 21;5:15. doi: 10.1186/s40425-017-0214-9. eCollection 2017. J Immunother Cancer. 2017. PMID: 28239468 Free PMC article.
-
Exacerbation of autoimmune hemolytic anemia induced by the first dose of programmed death-1 inhibitor pembrolizumab: a case report.Invest New Drugs. 2018 Jun;36(3):509-512. doi: 10.1007/s10637-018-0561-5. Epub 2018 Jan 16. Invest New Drugs. 2018. PMID: 29340837
-
Immunotherapy-associated Autoimmune Hemolytic Anemia.Hematol Oncol Clin North Am. 2022 Apr;36(2):365-380. doi: 10.1016/j.hoc.2021.11.002. Hematol Oncol Clin North Am. 2022. PMID: 35339260 Review.
-
Efficacy and safety evaluation of frontline immunotherapy combinations in advanced esophageal squamous cell carcinoma: a network meta-analysis highlighting the value of PD-L1 expression positivity scores.Front Immunol. 2024 Jul 10;15:1414753. doi: 10.3389/fimmu.2024.1414753. eCollection 2024. Front Immunol. 2024. PMID: 39050848 Free PMC article.
References
-
- Morgan E, Soerjomataram I, Rumgay H, et al. . The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–58.e2. - PubMed
-
- Sun JM, Shen L, Shah MA, et al. . Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–71. - PubMed
-
- Doki Y, Ajani JA, Kato K, et al. . Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

