Appendicectomy plus standard medical therapy versus standard medical therapy alone for maintenance of remission in ulcerative colitis (ACCURE): a pragmatic, open-label, international, randomised trial
- PMID: 40228513
- PMCID: PMC12062198
- DOI: 10.1016/S2468-1253(25)00026-3
Appendicectomy plus standard medical therapy versus standard medical therapy alone for maintenance of remission in ulcerative colitis (ACCURE): a pragmatic, open-label, international, randomised trial
Abstract
Background: The appendix might have an immunomodulatory role in ulcerative colitis. Appendicectomy has been suggested as a potentially therapeutic intervention to maintain remission in ulcerative colitis. We aimed to evaluate the clinical effectiveness of laparoscopic appendicectomy in maintaining remission in patients with ulcerative colitis.
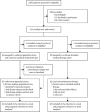
Methods: We did a pragmatic, open-label, international, randomised controlled superiority trial in 22 centres across the Netherlands, Ireland, and the UK. Patients with established ulcerative colitis who were in remission but had been treated for disease relapse within the preceding 12 months were randomly assigned (1:1) to undergo appendicectomy plus continued maintenance medical therapy (intervention group) or to continue maintenance medical therapy alone (control group). Randomisation was done with a central, computer-generated allocation concealment, stratified by disease extent. Patients and treating physicians were unmasked to group allocation. The prespecified primary outcome was the proportion of patients with a disease relapse within 1 year, predefined as a total Mayo score of 5 or higher with an endoscopic subscore of 2 or 3, or, in absence of endoscopy, based on a centrally independent masked review by a critical event committee as an exacerbation of abdominal symptoms (eg, elevated stool frequency subscore of ≥1 point from baseline) with a rectal bleeding subscore of ≥1 or faecal calprotectin level above 150 μg/g or necessitating treatment intensification other than mesalazine. Analyses were done on an intention-to-treat principle. This trial is complete and was registered with the Netherlands Trial Register (NTR2883) and ISRCTN (ISRCTN60945764).
Findings: Between Sept 20, 2012, and Sept 21, 2022, 1386 patients were screened. 201 patients were randomly assigned to the appendicectomy group (n=101) or the control group (n=100). After exclusion of four patients due to eligibility violations (three had active disease and one received biological agents at time of randomisation), 99 patients in the appendicectomy group and 98 patients in the control group were included in the intention-to-treat analyses. The 1-year relapse rate was significantly lower in the appendicectomy group than in the control group (36 [36%] of 99 patients vs 55 [56%] of 98 patients; relative risk 0·65 [95% CI 0·47-0·89]; p=0·005; adjusted p=0·002). Adverse events occurred in 11 (11%) of 96 patients in the appendicectomy group and 10 (10%) of 101 patients in the control group. The most frequently reported adverse events were postoperative temporary self-limiting abdominal pain in the appendicectomy group (three [3%] patients) and skin rash in the control group (three [3%] patients). Two cases (2%) of low-grade appendiceal mucinous neoplasm were incidentally found in resected appendix specimens in the appendicectomy group. Serious adverse events occurred in two (2%) of 96 patients who underwent appendicectomy and none in the control group. There were no deaths.
Interpretation: Appendicectomy is superior to standard medical therapy alone in maintaining remission in patients with ulcerative colitis.
Funding: Fonds Nuts-Ohra and National Institute for Health Research Efficacy and Mechanism Evaluation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests NA reports receiving consulting fees from Pfizer; presentation fees from Pfizer, Lilly, Takeda, and AbbVie; and travel support from Tillotts Pharma. WAB reports received speaker fees from Applied Medical and Johnson & Johnson. JPYB reports receiving funding from the National Institute for Health and Social Care Research (NIHR) Efficacy and Mechanism Evaluation (EME) programme for the conduct of the ACCURE-UK 2 study, the UK part of the ACCURE trial. CJB reports receiving funding from Nuts-Ohra (FNO 1202–008), the Netherlands part of the ACCURE trial; and received speakers fees from Takeda, Janssen, and Tillotts Pharma. RC reports receiving speaker fees from Takeda, Falk, Lilly, AbbVie, and Galapagos; and travel support from AMC Amsterdam to attend the ACCURE meeting. RMPHC reports serving as a proctor for Intuitive Surgical. RJD reports serving as the current chair of the data monitoring and ethics committee for the NIHR funded MEErKAT trial in the UK. GRD’H reports receiving grants from Pfizer, Takeda, AbbVie, Eli Lilly, BMS, and Alimentiv; consulting fees from AbbVie, Agomab, Alimentiv, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Index Pharmaceuticals, GlaxoSmithKline, Pfizer, Johnson & Johnson, Polpharma, Procise Diagnostics, Prometheus Laboratories, Prometheus Biosciences, and Ventyx; payment for lectures from AbbVie, Arena, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli Lilly, Johnson & Johnson, Pfizer, and Takeda; and travel support from Eli Lilly and Pfizer; and participation on data safety monitoring boards or advisory boards for Galapagos, AstraZeneca, and Seres Health. MD reports receiving grants from Galapogos–Alfasigma, Pfizer, Bristol Myers Squibb, Janssen, Celltrion, and Takeda; and received consulting fees from AbbVie, Galapagos–Alfasigma, Bristol Myers Squibb, Janssen, Celltrion and Takeda; and received speakers fees from Bristol Myers Squibb, Galapagos–Alfasigma, Takeda, Janssen, and Dr Falk. GD reports receiving grants from MSD, Pfizer, Janssen, AbbVie, Abbott, Takeda, Galapogos–Alfa Sigma, Eli Lilly, Celltrion and Amgen; speakers honorarium from AbbVie, Dr Falk, and Takeda. SCMF reports receiving speakers fee from Takeda, AbbVie, and Ferring; received support for attendance at educational conferences from Tillotts Pharma and Takeda. KH reports funding from the NIHR and the British Heart Foundation; and serves in unpaid roles on advisory boards for King's College London and the University of Oxford. GM reports receiving investigator grants from AstraZeneca, Janssen, Bristol Myers Squibb, Alimentiv, and Pfizer; received consulting fees from Pfizer, AbbVie, and Janssen; and honoraria for lectures and presentations from AbbVie and Takeda; serves on advisory boards for Pfizer, AbbVie, and Janssen; provided consultancy services for Alimentiv and Satisfai Health. TDP reports receiving grants from the NIHR for the ROSSINI-Platform Trial, ROSSINI 2 Extension Trial, and OCEAN trial, all of which are unrelated to this work; and received honoraria for lectures from MSD. CYP reports receiving grants from Next Generation Medicines and Perspectum; received consulting fees from Chemomab; and payment for expert testimony from Nationale Nederlanden. TR reports receiving institutional grants from AbbVie and Takeda; received consulting fees from AbbVie, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Ferring, Galapagos, Gilead, GSK, Heptares, Janssen, MonteRosa, MSD, Novartis, Numab, Pfizer, Roche, Sandoz, Takeda, Union Chimique Belge, and XAP Therapeutics; serves on the advisory board for Union Chimique Belge. IR reports receiving support from Tillotts Pharma UK for attending meeting. JPS reports receiving speaker fees from Pfizer, Alfasigma, Fresenius Kabi, AbbVie, Tillotts Pharma UK, Janssen-Cilag, Pharmacosmos UK, Takeda UK, and Bristol-Myers Squibb; received support for attending meetings and travel from AbbVie, Tillotts Pharma UK, Janssen-Cilag, and Celltrion; serves on advisory boards for Takeda, Galapagos, Dr Falk Pharma (UK), and AbbVie. BS reports being co-applicant on the NIHR grant for the UK part of the ACCURE trial, funded by the EME programme; received consulting fees from WPA for an advisory role unrelated to this research; and received honoraria for lectures from Arthrex. RW reports receiving speaker fees from Pfizer, AbbVie, and Ferring; and serves an unpaid role on the board of IBDREAM. MEW reports receiving research support from Boehringer Ingelheim and Hoffmann-LaRoche and a research contract from ExoBiologics. All other authors declare no competing interests.
Figures



Comment in
-
Appendicectomy: a novel treatment for ulcerative colitis?Lancet Gastroenterol Hepatol. 2025 Jun;10(6):497-498. doi: 10.1016/S2468-1253(25)00065-2. Epub 2025 Apr 11. Lancet Gastroenterol Hepatol. 2025. PMID: 40228514 No abstract available.
References
-
- Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402:571–584. - PubMed
-
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. - PubMed
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. - PubMed
-
- Bernklev T, Jahnsen J, Lygren I, Henriksen M, Vatn M, Moum B. Health-related quality of life in patients with inflammatory bowel disease measured with the short form-36: psychometric assessments and a comparison with general population norms. Inflamm Bowel Dis. 2005;11:909–918. - PubMed
-
- Nordin K, Påhlman L, Larsson K, Sundberg-Hjelm M, Lööf L. Health-related quality of life and psychological distress in a population-based sample of Swedish patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37:450–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

