Combating Concomitant Bacterial and Fungal Infections via Codelivery of Nitric Oxide and Fluconazole
- PMID: 40228797
- PMCID: PMC12022954
- DOI: 10.1021/acsami.5c00174
Combating Concomitant Bacterial and Fungal Infections via Codelivery of Nitric Oxide and Fluconazole
Abstract
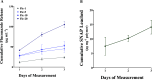
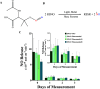
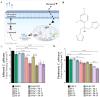
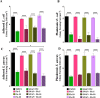
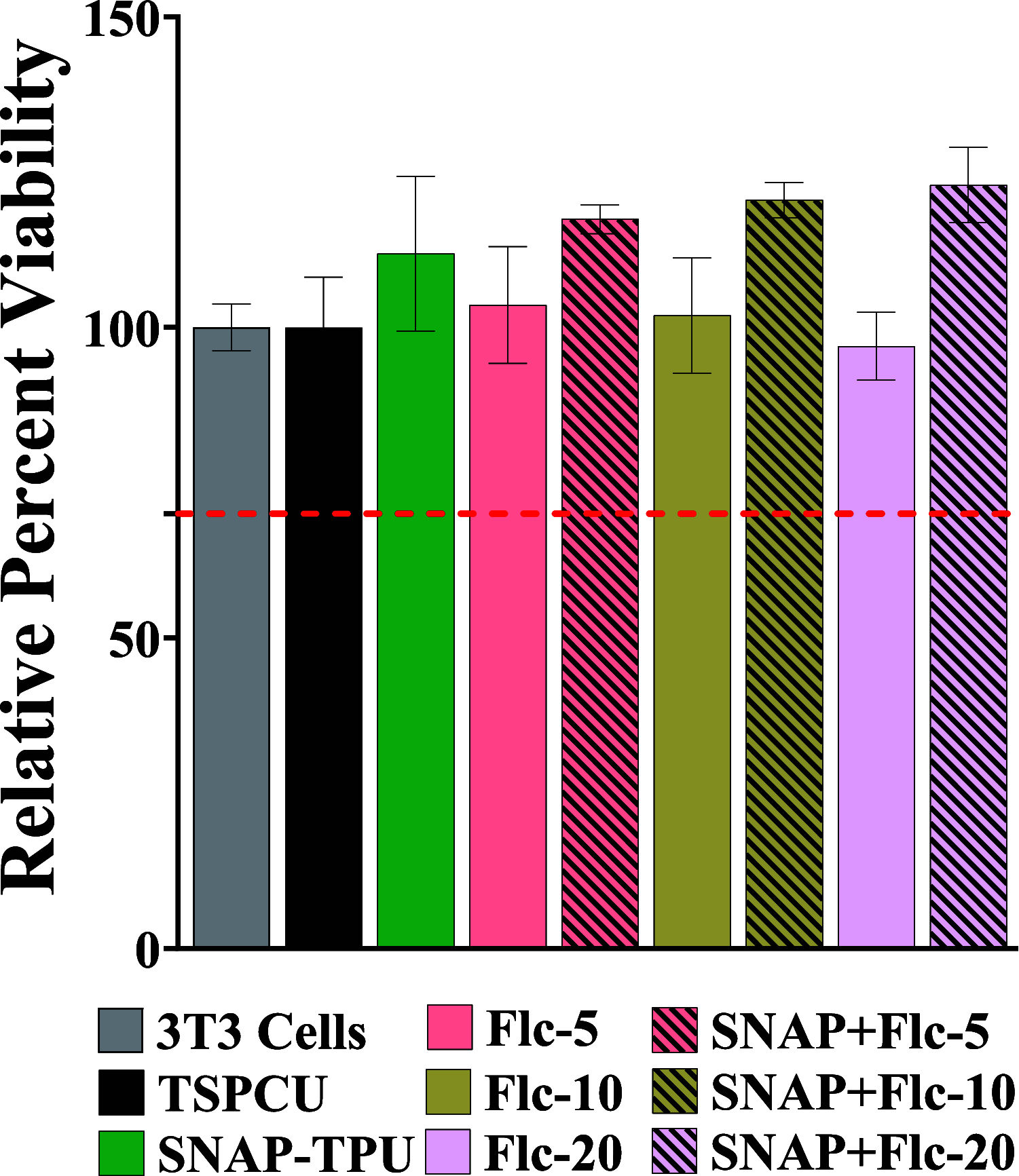
Device-associated infections are a major challenge for healthcare and cause patient morbidity and mortality as well as pose a significant economic burden. Infection-causing bacteria and fungi are equally notorious and responsible for biofilm formation and the development of antibiotic and antifungal-resistant strains. Biomaterials resisting bacterial and fungal adhesion can address device-associated infections more safely and efficiently than conventional systemic antibiotic therapies. Herein, we present a combination of potent antibacterial nitric oxide (NO) with antifungal fluconazole codelivery system from a polymeric matrix to combat bacterial and fungal infections simultaneously. The NO donor S-nitroso-N-acetyl-penicillamine (SNAP)-blended low-water-uptake polycarbonate urethane (TSPCU) was dip-coated with high-water-uptake polyether urethane (TPU) containing fluconazole to have an antibacterial and antifungal surface. The composites were characterized for surface wettability and coating stability using water contact angle (WCA) analysis. The real-time NO release (72 h) was evaluated using a chemiluminescence-based nitric oxide analyzer which showed physiologically relevant levels of NO released. The composites released fluconazole for 72 h under physiological conditions. Antibacterial analysis demonstrated a > 3-log reduction of viable Staphylococcus aureus and >2-log reduction of viable Escherichia coli compared to controls. The antifungal evaluation resulted in ∼98% reduction in adhered and ∼92% reduction in planktonic Candida albicans. The SNAP-fluconazole composites also showed biocompatibility against mouse fibroblast cells. This novel preventative strategy to combat bacterial and fungal infections may offer a promising tool for further translational research.
Keywords: antibacterial; antifungal; drug delivery; fluconazole; hospital-acquired infections; nitric oxide.
Conflict of interest statement
The authors declare the following competing financial interest(s): Hitesh Handa and Elizabeth J. Brisbois are co-founders and maintain a financial interest in Nytricx, Inc., a company investigating nitric oxide as a biomedical therapeutic for medical devices.
Figures


Similar articles
-
A Synergistic New Approach Toward Enhanced Antibacterial Efficacy via Antimicrobial Peptide Immobilization on a Nitric Oxide-Releasing Surface.ACS Appl Mater Interfaces. 2021 Sep 22;13(37):43892-43903. doi: 10.1021/acsami.1c08921. Epub 2021 Sep 13. ACS Appl Mater Interfaces. 2021. PMID: 34516076
-
Investigation of the susceptibility of clinical infection loads to nitric oxide antibacterial treatment.Nitric Oxide. 2025 Feb;154:19-28. doi: 10.1016/j.niox.2024.11.003. Epub 2024 Nov 17. Nitric Oxide. 2025. PMID: 39561942
-
A multi-defense strategy: Enhancing bactericidal activity of a medical grade polymer with a nitric oxide donor and surface-immobilized quaternary ammonium compound.Acta Biomater. 2017 Aug;58:421-431. doi: 10.1016/j.actbio.2017.05.061. Epub 2017 Jun 1. Acta Biomater. 2017. PMID: 28579540 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
[Advances in the study of synergistic effect of anti-biofilm agents].Yao Xue Xue Bao. 2012 Mar;47(3):339-45. Yao Xue Xue Bao. 2012. PMID: 22645757 Review. Chinese.
References
-
- Ahmadi M. S.; Lee H. H.; Sanchez D. A.; Friedman A. J.; Tar M. T.; Davies K. P.; Nosanchuk J. D.; Martinez L. R. Sustained nitric oxide-releasing nanoparticles induce cell death in Candida albicans yeast and hyphal cells, preventing biofilm formation in vitro and in a rodent central venous catheter model. Antimicrob. Agents Chemother. 2016, 60 (4), 2185–2194. 10.1128/AAC.02659-15. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

