Optimization and Characterization of N-Acetamide Indoles as Antimalarials That Target PfATP4
- PMID: 40228810
- PMCID: PMC12035806
- DOI: 10.1021/acs.jmedchem.5c00614
Optimization and Characterization of N-Acetamide Indoles as Antimalarials That Target PfATP4
Abstract
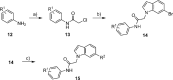
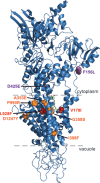
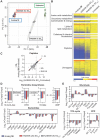
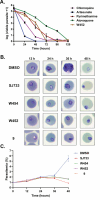
To discover new antimalarials, a screen of the Janssen Jumpstarter library against Plasmodium falciparum uncovered the N-acetamide indole hit class. The structure-activity relationship of this chemotype was defined and culminated in the optimized frontrunner analog WJM664, which exhibited potent asexual stage activity and high metabolic stability. Resistant selection and whole-genome sequencing revealed mutations in PfATP4, which was validated as the target by showing that analogs exhibited reduced potency against parasites with resistance-conferring mutations in PfATP4, a metabolomic signature similar to that of the PfATP4 inhibitor KAE609, and inhibition of Na+-dependent ATPase activity consistent with on-target inhibition of PfATP4. WJM664 inhibited gamete development and blocked parasite transmission to mosquitoes but exhibited low efficacy in aPlasmodium berghei mouse model, which was attributed to ATP4 species differentiation and its moderate systemic exposure. Optimization of these attributes is required for N-acetamide indoles to be pursued for development as a curative and transmission-blocking therapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Ashley E. A.; Dhorda M.; Fairhurst R. M.; Amaratunga C.; Lim P.; Suon S.; Sreng S.; Anderson J. M.; Mao S.; Sam B.; Sopha C.; Chuor C. M.; Nguon C.; Sovannaroth S.; Pukrittayakamee S.; Jittamala P.; Chotivanich K.; Chutasmit K.; Suchatsoonthorn C.; Runcharoen R.; Hien T. T.; Thuy-Nhien N. T.; Thanh N. V.; Phu N. H.; Htut Y.; Han K. T.; Aye K. H.; Mokuolu O. A.; Olaosebikan R. R.; Folaranmi O. O.; Mayxay M.; Khanthavong M.; Hongvanthong B.; Newton P. N.; Onyamboko M. A.; Fanello C. I.; Tshefu A. K.; Mishra N.; Valecha N.; Phyo A. P.; Nosten F.; Yi P.; Tripura R.; Borrmann S.; Bashraheil M.; Peshu J.; Faiz M. A.; Ghose A.; Hossain M. A.; Samad R.; Rahman M. R.; Hasan M. M.; Islam A.; Miotto O.; Amato R.; MacInnis B.; Stalker J.; Kwiatkowski D. P.; Bozdech Z.; Jeeyapant A.; Cheah P. Y.; Sakulthaew T.; Chalk J.; Intharabut B.; Silamut K.; Lee S. J.; Vihokhern B.; Kunasol C.; Imwong M.; Tarning J.; Taylor W. J.; Yeung S.; Woodrow C. J.; Flegg J. A.; Das D.; Smith J.; Venkatesan M.; Plowe C. V.; Stepniewska K.; Guerin P. J.; Dondorp A. M.; Day N. P.; White N. J. Spread of Artemisinin resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2014, 371 (5), 411–423. 10.1056/NEJMoa1314981. - DOI - PMC - PubMed
-
- Uwimana A.; Legrand E.; Stokes B. H.; Ndikumana J.-L. M.; Warsame M.; Umulisa N.; Ngamije D.; Munyaneza T.; Mazarati J.-B.; Munguti K.; Campagne P.; Criscuolo A.; Ariey F.; Murindahabi M.; Ringwald P.; Fidock D. A.; Mbituyumuremyi A.; Menard D. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. 10.1038/s41591-020-1005-2. - DOI - PMC - PubMed
-
- Jiménez-Díaz M. B.; Ebert D.; Salinas Y.; Pradhan A.; Lehane A. M.; Myrand-Lapierre M.-E.; O’Loughlin K. G.; Shackleford D. M.; Justino de Almeida M.; Carrillo A. K.; et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (50), E545510.1073/pnas.1414221111. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

