Total Synthesis of Kanamycins
- PMID: 40229233
- PMCID: PMC12238912
- DOI: 10.1002/chem.202500685
Total Synthesis of Kanamycins
Abstract
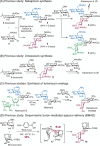
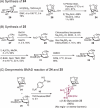
Structurally modified aminoglycosides, such as kanamycin, have shown promise as antibiotics and premature termination codon read-through drugs to fight against drug-resistant bacteria and to treat genetic diseases, respectively. Therefore, research on developing and discovering aminoglycoside antibiotics has recently increased. However, synthetic strategies for controllably positioning the two 1,2-cis-glycoside moieties on the symmetrical structure of kanamycin have not yet been established. Herein, we report on a novel route for the total synthesis of kanamycins A and B via regio- and stereoselective introduction of 1,2-cis-glycosidic linkages as the key step. We successfully synthesized α(1,6)-linked glycoside using a 1,2-anhydro donor and 2-deoxy-myo-inositol 1,3,5-orthoformate acceptor in the presence of a boronic acid catalyst via desymmetric boron-mediated aglycon delivery. In addition, we also synthesized α(1,4)-linked glycoside using its corresponding trichloroacetimidate donor.
Keywords: boron‐mediated aglycon delivery; desymmetrization; glycosylation; kanamycin; total synthesis.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

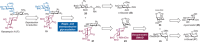

References
-
- Umezawa H., Ueda M., Maeda K., Yagishita K., Kondo S., Okami Y., Utahara R., Osato Y., Nitta K., Takeuchi T., J. Antibiot. 1957, 10, 181. - PubMed
-
- Umezawa H., Adv. Carbohydr. Chem. Biochem. 1974, 30, 183. - PubMed
-
- Kawaguchi H., Naito T., Nakagawa S., Fujisawa K.‐I., J. Antibiot. 1972, 25, 695. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

