A targetable antioxidant defense mechanism to EZH2 inhibitors enhances tumor cell vulnerability to ferroptosis
- PMID: 40229247
- PMCID: PMC11997205
- DOI: 10.1038/s41419-025-07607-y
A targetable antioxidant defense mechanism to EZH2 inhibitors enhances tumor cell vulnerability to ferroptosis
Abstract
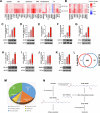
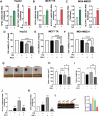
Epigenetic changes are present in all human cancers and are responsible for switching on or off genes, thus controlling tumor cell transcriptome. These changes occur through DNA methylation, histone modifiers and readers, chromatin remodelers, and microRNAs. The histone H3 methyl-transferase EZH2 gene is overexpressed in several cancer types, including adrenocortical carcinoma (ACC), a rare cancer still lacking a targeted therapy. EZH2 inhibitors (EZH2i) have been tested in several clinical trials, but their effectiveness was limited by the toxic effects of the therapeutic doses. We tested several EZH2i on ACC cells, and observed a significant reduction in cell growth only with doses much higher than those required to prevent H3 methylation. We found that all tested EZH2i doses affected lipid metabolism genes, ROS, and glutathione production. Transcript changes correlated with metabolic data, which suggested the effects of EZH2i on ferroptosis. We found that EZH2i dose-dependently increased SLC7A11/glutathione axis and glutathione peroxidase-4 (GPX4), required to counteract lipid peroxidation and ferroptosis. A GPX4 inhibitor synergized with EZH2i, making low doses - which otherwise do not affect cell viability - able to significantly reduce ACC cell growth in vitro and in vivo. Importantly, we found that the anti-ferroptosis defense mechanism induced by EZH2i is a common response for several aggressive tumor phenotypes, uncovering a general co-targetable mechanism that could limit EZH2i effectiveness. Correcting this antioxidant response by ferroptosis inducers may be a new combination therapy that will easily find clinical applications.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Ghosh C, Hu J, Kebebew E. Advances in translational research of the rare cancer type adrenocortical carcinoma. Nat Rev Cancer. 2023;2023:1–20. - PubMed
-
- Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, et al. Mitotane inhibits sterol-O-acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156:3895–908. - PubMed
-
- LaPensee CR, Mann JE, Rainey WE, Crudo V, Hunt SW, Hammer GD. ATR-101, a selective and potent inhibitor of acyl-CoA acyltransferase 1, induces apoptosis in H295R adrenocortical cells and in the adrenal cortex of dogs. Endocrinology. 2016;157:1775–88. - PubMed
-
- Smith DC, Kroiss M, Kebebew E, Habra MA, Chugh R, Schneider BJ, et al. A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma. Investig N Drugs. 2020;38:1421–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

