SPI1 activates mitochondrial unfolded response signaling to inhibit chondrocyte senescence and relieves osteoarthritis
- PMID: 40229258
- PMCID: PMC11997156
- DOI: 10.1038/s41413-025-00421-4
SPI1 activates mitochondrial unfolded response signaling to inhibit chondrocyte senescence and relieves osteoarthritis
Abstract
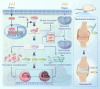
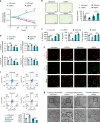
Chondrocyte senescence is a critical pathological hallmark of osteoarthritis (OA). Aberrant mechanical stress is considered a pivotal determinant in chondrocyte aging; however, the precise underlying mechanism remains elusive. Our findings demonstrate that SPI1 plays a significant role in counteracting chondrocyte senescence and inhibiting OA progression. SPI1 binds to the PERK promoter, thereby promoting its transcriptional activity. Importantly, PERK, rather than GCN2, facilitates eIF2α phosphorylation, activating the mitochondrial unfolded protein response (UPRmt) and impeding chondrocyte senescence. Deficiency of SPI1 in mechanical overload-induced mice leads to diminished UPRmt activation and accelerated OA progression. Intra-articular injection of adenovirus vectors overexpressing SPI1 and PERK effectively mitigates cartilage degeneration. In summary, our study elucidates the crucial regulatory role of SPI1 in the pathogenesis of chondrocyte senescence by activating UPRmt signaling through PERK, which may present a novel therapeutic target for treating OA. SPI1 alleviates the progression of OA by inhibiting mechanical stress-induced chondrocyte senescence through mitochondrial UPR signaling.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

