ICAM-1/CD18-mediated sequestration of parasitized phagocytes in cortical capillaries promotes neuronal colonization by Toxoplasma gondii
- PMID: 40229286
- PMCID: PMC11997185
- DOI: 10.1038/s41467-025-58655-z
ICAM-1/CD18-mediated sequestration of parasitized phagocytes in cortical capillaries promotes neuronal colonization by Toxoplasma gondii
Abstract
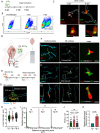
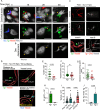
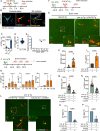
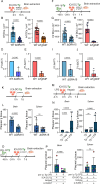
Microbial translocation across the blood-brain barrier (BBB) is a prerequisite for colonization of the central nervous system. The obligate intracellular parasite Toxoplasma gondii chronically infects the brain parenchyma of humans and animals, in a remarkably stealthy fashion. We investigated the mechanisms of BBB traversal by T. gondii (genotypes I, II, III) and T. gondii-infected leukocytes, using intracarotid arterial delivery into the cerebral circulation of mice. Unexpectedly, parasitized dendritic cells (DCs) and other peripheral blood mononuclear cells were found to persistently sequester within cortical capillaries. Post-replicative egress of T. gondii from sequestered DCs was followed by rapid parasite localization within cortical neurons. Infection-induced microvascular inflammation dramatically elevated the sequestration of parasitized DCs, while treatments targeting the ICAM-1/CD18 leukocyte adhesion axis with blocking antibodies strongly reverted sequestration. The parasite effectors TgWIP and GRA15, known to promote leukocyte hypermigration and inflammatory activation, further increased both the capillary sequestration of infected DCs and cerebral parasite loads in a strain-dependent manner. These findings reveal that the sequestration of parasitized leukocytes in cortical capillaries, with subsequent BBB traversal following parasite egress, provides a mechanism for T. gondii's rapid access to cortical neurons during primary infection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that no competing interests exist.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

