A systems biology approach to define SARS-CoV-2 correlates of protection
- PMID: 40229322
- PMCID: PMC11997207
- DOI: 10.1038/s41541-025-01103-2
A systems biology approach to define SARS-CoV-2 correlates of protection
Abstract
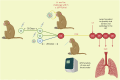
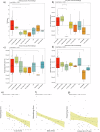
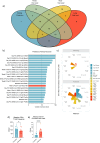
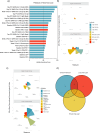
Correlates of protection (CoPs) for SARS-CoV-2 have yet to be sufficiently defined. This study uses the machine learning platform, SIMON, to accurately predict the immunological parameters that reduced clinical pathology or viral load following SARS-CoV-2 challenge in a cohort of 90 non-human primates. We found that anti-SARS-CoV-2 spike antibody and neutralising antibody titres were the best predictors of clinical protection and low viral load in the lung. Since antibodies to SARS-CoV-2 spike showed the greatest association with clinical protection and reduced viral load, we next used SIMON to investigate the immunological features that predict high antibody titres. It was found that a pre-immunisation response to seasonal beta-HCoVs and a high frequency of peripheral intermediate and non-classical monocytes predicted low SARS-CoV-2 spike IgG titres. In contrast, an elevated T cell response as measured by IFNγ ELISpot predicted high IgG titres. Additional predictors of clinical protection and low SARS-CoV-2 burden included a high abundance of peripheral T cells. In contrast, increased numbers of intermediate monocytes predicted clinical pathology and high viral burden in the throat. We also conclude that an immunisation strategy that minimises pathology post-challenge did not necessarily mediate viral control. This would be an important finding to take forward into the development of future vaccines aimed at limiting the transmission of SARS-CoV-2. These results contribute to SARS-CoV-2 CoP definition and shed light on the factors influencing the success of SARS-CoV-2 vaccination.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Author S.L. serves as an Associate Editor of this journal and had no role in the peer-review or decision to publish this manuscript. All other authors declare no financial or non-financial competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

