Effects of Uro-Vaxom vs. placebo on the urinary tract microbiome in individuals with spinal cord injury in a randomized controlled pilot trial (Uro-Vaxom pilot)
- PMID: 40229352
- PMCID: PMC11997090
- DOI: 10.1038/s41598-025-96939-y
Effects of Uro-Vaxom vs. placebo on the urinary tract microbiome in individuals with spinal cord injury in a randomized controlled pilot trial (Uro-Vaxom pilot)
Abstract
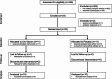
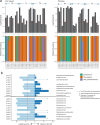
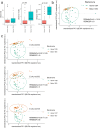
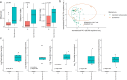
Individuals with spinal cord injury/disease (SCI/D) have a high incidence of urinary tract infections (UTI). This randomized controlled pilot trial investigated the effect of an immunomodulator (Uro-Vaxom) versus a placebo on the urinary tract microbiome of individuals with SCI/D to inform the design of a larger trial. Twenty participants with SCI/D undergoing primary rehabilitation were randomized to receive either Uro-Vaxom or a placebo for three months (ClinicalTrials.gov NCT04049994 08/08/2019). Urine was collected at baseline, immediately post-treatment, and three months post-treatment. DNA was extracted and sequenced using full-length 16 S rRNA using Oxford Nanopore technology. Internal controls were added for absolute abundance estimation. There were 10 participants in Uro-Vaxom and 10 in placebo analyzed. The prevalence of Escherichia coli was lower in the Uro-Vaxom group (2/10) compared to the placebo group (5/10) post-treatment, although this difference was not statistically significant. Significant alpha and beta diversity differences were associated with the microbial load, sex, and voiding method. Uro-Vaxom showed potential in reducing E. coli prevalence during the treatment period, but this result requires validation in a larger trial. Future trials should consider the baseline microbial load and optimal timing of intervention to ensure that the observed effects are attributable to immunomodulation.
Keywords: Full length 16S rRNA; Immunomodulation; Spinal cord injury; Urinary tract infections; Uro-Vaxom.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

