Escherichia coli type I toxin TisB exclusively controls proton depolarization following antibiotic induced DNA damage
- PMID: 40229382
- PMCID: PMC11997105
- DOI: 10.1038/s41598-025-96136-x
Escherichia coli type I toxin TisB exclusively controls proton depolarization following antibiotic induced DNA damage
Abstract
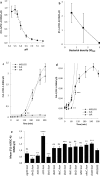
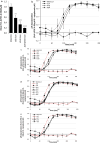
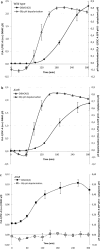
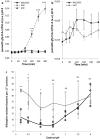
Bacterial toxin-antitoxin (TA) systems are genetic loci where the antitoxin gene product helps to control the expression or activity of the toxin gene product. Type I TA systems typically produce hydrophobic peptides that often localize to the inner membrane of bacteria. These amphipathic peptides can then potentially affect ion flows across the inner membrane. Here, we show that several type I toxins from Escherichia coli can affect depolarization, whereas tisB exclusively controls the depolarization of the proton gradient. tisB has been linked to persister cell formation following treatment with the antibiotic ciprofloxacin and tisB-istR has been implicated in the control of proton depolarization following treatment with ofloxacin. These results suggest that tisB could initiate the formation of persister cells by fully dissipating the proton gradient and that most of the electrical gradient greatly limiting ATP production following antibiotic-induced DNA damage.
Keywords: E. coli; Antibiotics; DNA damage; Membrane depolarization; TisB; Type I toxin antitoxin systems.
© 2025. The Author(s).
Conflict of interest statement
Delcaration. Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Protein aggregation is a consequence of the dormancy-inducing membrane toxin TisB in Escherichia coli.mSystems. 2024 Nov 19;9(11):e0106024. doi: 10.1128/msystems.01060-24. Epub 2024 Oct 8. mSystems. 2024. PMID: 39377584 Free PMC article.
-
Relevance of charged and polar amino acids for functionality of membrane toxin TisB.Sci Rep. 2024 Oct 3;14(1):22998. doi: 10.1038/s41598-024-73879-7. Sci Rep. 2024. PMID: 39362964 Free PMC article.
-
Two regulatory RNA elements affect TisB-dependent depolarization and persister formation.Mol Microbiol. 2017 Mar;103(6):1020-1033. doi: 10.1111/mmi.13607. Epub 2017 Jan 13. Mol Microbiol. 2017. PMID: 27997707
-
The toxin-antitoxin system tisB-istR1: Expression, regulation, and biological role in persister phenotypes.RNA Biol. 2012 Dec;9(12):1513-9. doi: 10.4161/rna.22578. Epub 2012 Oct 23. RNA Biol. 2012. PMID: 23093802 Review.
-
Toxin-antitoxin systems influence biofilm and persister cell formation and the general stress response.Appl Environ Microbiol. 2011 Aug 15;77(16):5577-83. doi: 10.1128/AEM.05068-11. Epub 2011 Jun 17. Appl Environ Microbiol. 2011. PMID: 21685157 Free PMC article. Review.
References
-
- Yamaguchi, Y. & Inouye, M. Regulation of growth and death in escherichia coli by toxin-antitoxin systems. Nat. Rev. Microbiol.9, 779–790. 10.1038/nrmicro2651 (2011). - PubMed
-
- Darfeuille, F., Unoson, C., Vogel, J. & Wagner, E. G. H. An antisense RNA inhibits translation by competing with standby ribosomes. Mol. Cell26, 381–392. 10.1016/j.molcel.2007.04.003 (2007). - PubMed
-
- Kawano, M., Oshima, T., Kasai, H. & Mori, H. Molecular characterization of long direct repeat (LDR) sequences expressing a stable mRNA encoding for a 35-amino-acid cell-killing peptide and a cis-encoded small antisense RNA in escherichia coli. Mol. Microbiol.45, 333–349. 10.1046/j.1365-2958.2002.03042.x (2002). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

